Acid and Base Molecules
What is an Acid?
An acid is something that has a pH less than 7.0 . The chemistry definition of an acid is: "a substance that donates a hydrogen ion (H+) to another substance, which is called a base. (Or give out hydrogen ions when dissolved in water.)"
What are the properties of acids?
1) Acids taste sour when they are eaten can sting the skin when they are touched
2) Acids can corrode (or eat away at) metals and skin.
3) Acids can be used as a reactant during electrolysis.
4) Acids will turn blue litmus paper red.
Common Acids
| Hydrochloric Acid | Lactic Acid |
| Acetic Acid | Citric Acid |
| Carbonic Acid | Boric Acid |
| Nitric Acid | Sulfuric Acid |
What is a Base?
A base (in chemistry) means having a pH (on the pH scale) of 8-14.It is a substance that can accept protons. A base that is dissolved in water is called an alkali.
What are the properties of bases?
Bases have a bitter taste (opposed to sour taste of acids)
Bases fee; slimy, or soapy feel on fingers
Bases turn red litmus paper blue
If mixed with an acid, bases will reduce the pH value.
If touched, it may cause a skin irritation.
Common Bases
| Ammonia (Ammonium hydroxide) | Sodium bicarbonate |
| Sodium Hydroxide | Potassium Hydroxide |
| Calcium Hydroxide | Magnesium Hydroxide |
What is pH?
The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic).
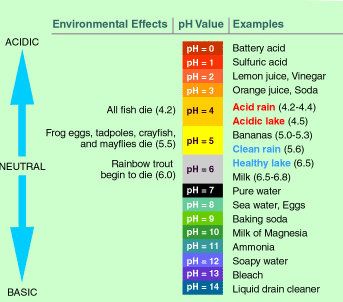
Typical acid rain has a pH value of 4.0. A decrease in pH values from 5.0 to 4.0 means that the acidity is 10 times greater.
How pH is Measured
There are many high-tech devices that are used to measure pH in laboratories. One easy way that you can measure pH is with a strip of litmus paper. When you touch a strip of litmus paper to something, the paper changes color depending on whether the substance is acidic or basic. If the paper turns red, the substance is acidic, and if it turns blue, the substance is basic.
Molecules of Life Resources
Molecules in the News
- Cannabidiol significantly reduces seizures in patients with severe form of epilepsy - May 18, 2018
- Mechanism for how alcohol increases cancer risk Jan 2018
- MitoQ a novel antioxidant makes old blood vessels seem young again April 19, 2018
- Spermidine-rich foods may prevent liver cancer, extend lifespan April 2017
- Peptide targeting senescent cells restores stamina in elderly miceMarch 2017
- Capsaicin, an active ingredient of pungent substances such as chilli or pepper, inhibits the growth of breast cancer cells Dec. 2016 com/news/354895-molecule-tissue-regeneration-russia-acegram/">Russian scientists speed up human tissue regeneration with supermolecule - August 6,2016
- Urolithin A Molecule --Pomegranate finally reveals its powerful anti-aging secret -- July 11, 2016
- --SEE MORE MOLECULES IN THE NEWS--