Chlorophyll Molecule
Chlorophyll Molecule Ball-and-Stick Model
To View the Chlorophyll Molecule in 3D --->>in 3D with Jsmol
Chlorophyll
Chlorophyll is a green pigment found in most plants, algae, and cyanobacteria. Its name is derived from Greek: chloros = green and phyllon = leaf. Chlorophyll absorbs light most strongly in the blue and red but poorly in the green portions of the electromagnetic spectrum, hence the green color of chlorophyll-containing tissues like plant leaves.[1]
Chlorophyll and photosynthesis Chlorophyll is vital for photosynthesis, which allows plants to obtain energy from light. Chlorophyll molecules are specifically arranged in and around pigment protein complexes called photosystems which are embedded in the thylakoid membranes of chloroplasts. In these complexes, chlorophyll serves two primary functions. The function of the vast majority of chlorophyll (up to several hundred per photosystem) is to absorb light and transfer that light energy by resonance energy transfer to a specific chlorophyll pair in the reaction center of the photosystems. Because of chlorophyll’s selectivity regarding the wavelength of light it absorbs, areas of a leaf containing the molecule will appear green. There are currently two accepted photosystem units, Photosystem II and Photosystem I, which have their own distinct reaction center chlorophylls, named P680 and P700, respectively.[2] These pigments are named after the wavelength (in nanometers) of their red-peak absorption maximum. The identity, function and spectral properties of the types of chlorophyll in each photosystem are distinct and determined by each other and the protein structure surrounding them. Once extracted from the protein into a solvent (such as acetone or methanol), these chlorophyll pigments can be separated in a simple paper chromatography experiment, and, based on the number of polar groups between chlorophyll a and chlorophyll b, will chemically separate out on the paper. The function of the reaction center chlorophyll is to use the energy absorbed by and transferred to it from the other chlorophyll pigments in the photosystems to undergo a charge separation, a specific redox reaction in which the chlorophyll donates an electron into a series of molecular intermediates called an electron transport chain. The charged reaction center chlorophyll (P680+) is then reduced back to its ground state by accepting an electron. In Photosystem II, the electron which reduces P680+ ultimately comes from the oxidation of water into O2 and H+ through several intermediates. This reaction is how photosynthetic organisms like plants produce O2 gas, and is the source for practically all the O2 in Earth's atmosphere. Photosystem I typically works in series with Photosystem II, thus the P700+ of Photosystem I is usually reduced, via many intermediates in the thylakoid membrane, by electrons ultimately from Photosystem II. Electron transfer reactions in the thylakoid membranes are complex, however, and the source of electrons used to reduce P700+ can vary. The electron flow produced by the reaction center chlorophyll pigments is used to shuttle H+ ions across the thylakoid membrane, setting up a chemiosmotic potential mainly used to produce ATP chemical energy, and those electrons ultimately reduce NADP+ to NADPH a universal reductant used to reduce CO2 into sugars as well as for other biosynthetic reductions.
Reaction center chlorophyll-protein complexes are capable of directly absorbing light and performing charge separation events without other chlorophyll pigments, but the absorption cross section (the likelihood of absorbing a photon under a given light intensity) is small. Thus, the remaining chlorophylls in the photosystem and antenna pigment protein complexes associated with the photosystems all cooperatively absorb and funnel light energy to the reaction center. Besides chlorophyll a, there are other pigments, called accessory pigments, which occur in these pigment-protein antenna complexes.
Chemical structure Chlorophyll is a chlorin pigment, which is structurally similar to and produced through the same metabolic pathway as other porphyrin pigments such as heme. At the center of the chlorin ring is a magnesium ion. The chlorin ring can have several different side chains, usually including a long phytol chain. There are a few different forms that occur naturally, but the most widely distributed form in terrestrial plants is chlorophyll a. The general structure of chlorophyll a was elucidated by Hans Fischer in 1940, and by 1960, when most of the stereochemistry of chlorophyll a was known, Robert Burns Woodward published a total synthesis of the molecule as then known.[3] In 1967, the last remaining stereochemical elucidation was completed by Ian Fleming,[4] and in 1990 Woodward and co-authors published an updated synthesis.[5] The different structures of chlorophyll are summarized below:
| Chlorophyll a | Chlorophyll b | Chlorophyll c1 | Chlorophyll c2 | Chlorophyll d | |
|---|---|---|---|---|---|
| Molecular formula | C55H72O5N4Mg | C55H70O6N4Mg | C35H30O5N4Mg | C35H28O5N4Mg | C54H70O6N4Mg |
| C3 group | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CHO |
| C7 group | -CH3 | -CHO | -CH3 | -CH3 | -CH3 |
| C8 group | -CH2CH3 | -CH2CH3 | -CH2CH3 | -CH=CH2 | -CH2CH3 |
| C17 group | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl | -CH=CHCOOH | -CH=CHCOOH | -CH2CH2COO-Phytyl |
| C17-C18 bond | Single | Single | Double | Double | Single |
| Occurrence | Universal | Mostly plants | Various algae | Various algae | cyanobacteria |
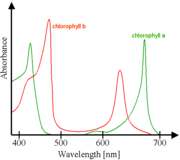 |
Absorbance spectra of free chlorophyll a (green) and b (red) in a solvent. The spectra of chlorophyll molecules are slightly modified in vivo depending on specific pigment-protein interactions.
References
- Speer, Brian R. (1997). "Photosynthetic Pigments" in UCMP Glossary (online). University of California, Berkeley Museum of Paleontology. Verified availability March 12, 2007.
- Green, 1984
- R. B. Woodward, W. A. Ayer, J. M. Beaton, F. Bickelhaupt, R. Bonnett, P. Buchschacher, G. L. Closs, H. Dutler, J. Hannah, F. P. Hauck.S, A. Langemann, E. Le Goff, W. Leimgruber, W. Lwowski, J. Sauer, Z. Valenta, and H. Volz (1960). "The total synthesis of chlorophyll". Journal of the American Chemical Society 82: 3800-3802.
- Ian Fleming (October 1967). "Absolute Configuration and the Structure of Chlorophyll". Nature 216: 151-152. doi:10.1038/216151a0.
- Robert Burns Woodward, William A. Ayer, John M. Beaton, Friedrich Bickelhaupt, Raymond Bonnett, Paul Buchschacher, Gerhard L. Closs, Hans Dutler, John Hannah, Fred P. Hauck, et al. (1990). "The total synthesis of chlorophyll a". Tetrahedron 46 (22): 7599-7659.
- Colorless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants Thomas Muller, Markus Ulrich, Karl-Hans Ongania, and Bernhard Krautler Angew. Chem. Int. Ed. 2007, 46, 8699 -8702 doi:10.1002/anie.200703587
- Gross, 1991
- Oregon University of Health & Sciences
- PDF review-Chlorophyll d: the puzzle resolved
- Light Absorption by Chlorophyll NIH book