Molecular Weight: 147.13
Isolectric point (pH) 3.22
Molecular Formula: C 5H9NO4
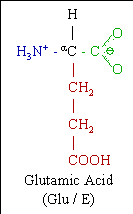
Glutamic acid or glutamate is one of the 20 most common natural amino acids. As its name indicates, it is acidic, with a carboxylic acid component to its side chain.
Glutamic acid is critical for proper cell function, but it is not considered an essential nutrient in humans because the body can manufacture it from simpler compounds. In addition to being one of the building blocks in protein synthesis, it is also important in rain function, as an excitatory neurotransmitter and as a precursor for the synthesis of GABA in GABAergic neurons. Glutamate activates both ionotropic and metabotropic glutamate receptors. The ionotropic ones being non-NMDA (AMPA and kainate) and NMDA receptors. Free glutamic acid cannot cross the blood-brain barrier in appreciable quantities; instead it is converted into L-glutamine, which the brain uses for fuel and protein synthesis.
The sodium salt of glutamic acid, monosodium glutamate (MSG) is responsible for one of the five basic tastes of the human sense of taste (umami), and MSG is extensively used as a food additive.