Chlorophyll Molecule - Chemical and Physical Properties
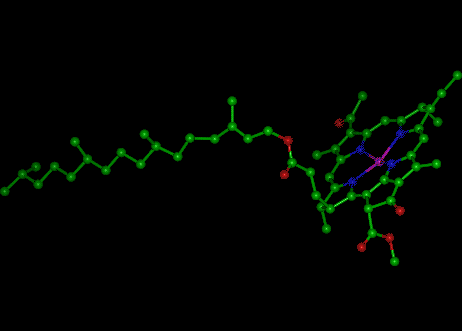
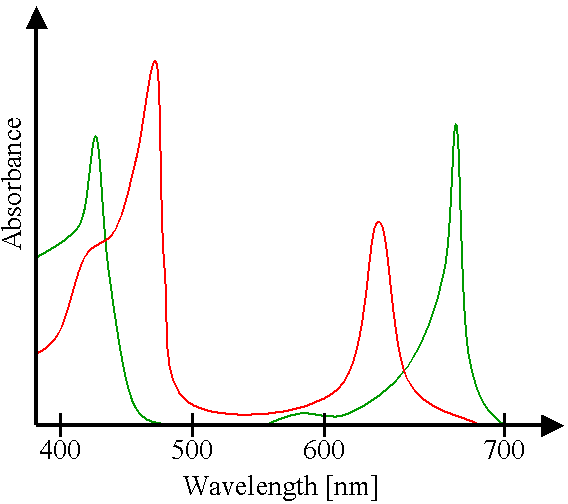
Chlorophyll is the green photosynthetic pigment present in chloroplasts which provides the energy necessary for photosynthesis. The intense green color of chlorophyll is due to its strong absorbencies in the red and blue regions of the electromagnetic spectrum, and because of these absorbencies the light it reflects and transmits appears green. It is capable of channeling the energy of sunlight into chemical energy through the process of photosynthesis. In this process the energy absorbed by chlorophyll transforms carbon dioxide and water into carbohydrates and oxygen.
For 3-D Structure of this image using Jsmol Click here
Chemical structure
Chlorophyll is a chlorin pigment, related to the porphyrin containing iron compound known as heme. At the center of the ring is a magnesium ion. The side chains vary somewhat between the different forms of chlorophyll found in different organisms - chlorophyll a is always present, but chlorophylls b and c also occur in various groups.
Absorbance of light of chlorophyll a(green) and b(red)
Forms of chlorophyll
Chlorophyll consists of two forms, A and B.
A: C66H72O5N4Mg
B: C66H70O6N4Mg
In both cases the magnesium atom is central in the molecule.
Function of chlorophyll
This green pigment is what gives green plants their colour. It is involved in photosynthesis by absorbing energy from visible light.