|
Why
is water such a good solvent?
|
[Water
Molecule is shown using the Jsmol] | To
rotate click left mouse button over image and drag cursor. Zoom with middle button...
Try
changing to CPK -- Click right mouse button in box--->
Style--->Scheme
-->CPK Use left mouse button to rotate
[Right
mouse button for more Options] | | |
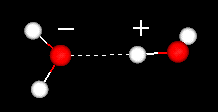
A water
molecule is formed when two atoms of hydrogen bond covalently with an atom
of oxygen. In a covalent bond electrons are shared between atoms. In water the
sharing is not equal. The oxygen atom attracts the electrons more strongly than
the hydrogen. This gives water an asymmetrical distribution of charge. Molecules
that have ends with partial negative and positive charges are known as polar molecules.
It is this polar property that allows water to separate polar solute molecules
and explains why water can dissolve so many substances.
Water
is a good solvent due to its polarity. The solvent properties of water are vital
in biology, because many biochemical reactions take place only within aqueous
solutions When
an ionic or polar compound enters water, it is surrounded by water molecules.
The relatively small size of water molecules typically allows many water molecules
to surround one molecule of solute. The partially negative dipoles of the water
are attracted to positively charged components of the solute, and vice versa for
the positive dipoles. An
example of an ionic solute is table salt.
| 
| Liquid
water has a partially ordered structure in which hydrogen
bonds are constantly being formed and breaking up. |
The strong hydrogen
bonds also give water a high cohesiveness and, consequently, surface tension.
This is evident when small quantities of water are put onto a nonsoluble surface
and the water stays together as drops. | 
