Botulinum toxin protein molecule
CRYSTAL STRUCTURE OF BOTULINUM NEUROTOXIN SEROTYPE A RCSB:3BTA
Structure of the botulinum toxins
Botulinum neurotoxin (BoNT) is one the most lethal biological toxins known and causes the disease know as botulism. The toxin is produced by the Gram-positive, obligate anaerobic bacterium Clostridium botulinum. BoNT is a zinc-dependent metalloprotease with a molecular mass of 150 kD. It elaborates eight antigenically distinguishable exotoxins (A, B, C1, C2, D, E, F and G). All of the eight exotoxins interfere with neural transmission by blocking the release of acetylcholine, the principal neurotransmitter at the neuromuscular junction, causing muscle paralysis. [1]
Botulinum toxins act at four different sites in the body: The neuromuscular junction, autonomic ganglia, postganglionic parasympathetic nerve endings and postganglionic sympathetic nerve endings that release acetylcholine.[2}
BoNTs are large neurotoxic proteins of ∼150 kDa that consist of a light chain (L-chain; 50 kDa) and a heavy chain (H-chain; 100 kDa) linked by a disulfide bond.. These two chains are linked by a single disulfide bond which plays an essential role during the entry of the metalloprotease chain in the cytosol [4]. As with tetanospasmin, the chains remain connected by a disulfide bond.
The function of L-chain is to cleave SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins that involve in the exocytosis of neurotransmitter whereas H-chain is responsible for binding of toxin with nerve terminal and translocating of L-chain into cytosol from synaptic vesicle [5]. The L-chain fragment of the cleaved toxin, on a molecular weight basis, becomes the most potent toxin found in nature. (see mechanism of action below).
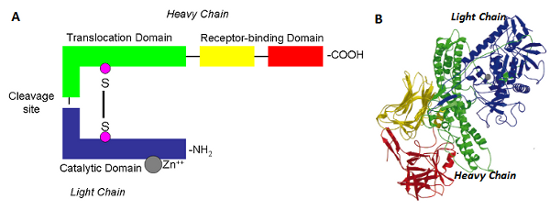
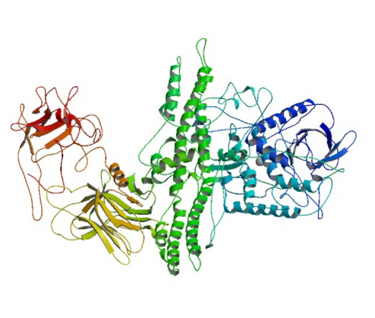
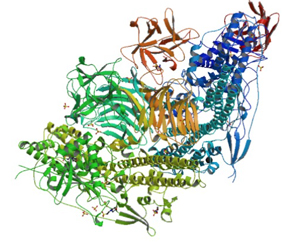
Figure above: Botulinum toxin structure A) schematic representation of BoNT, B) Crystal structure of BoNT/A - PDBcode:3BTA. Figure B - taken from [1]. The Light chain catalytic domain is coloured in blue. The heavy chain translocation domain is coloured in green, N-terminal and the C-terminal receptor binding domains are coloured in yellow and red respectively. The catalytic zinc is represented as a ball in gray. The colour code is similar for Figures 1A and 1B. Source of image and description from BioModels Database - EMBL- EBI and reference [6]
How is Botulinum Toxin protein resistant to proteolytic digestion?
When pure BoNT is exposed to the digestive conditions with acidic fluid and proteases, the BoNT easily degrades into inactive short peptides and thus the pure BoNT exerts the only weak or no oral toxicity. This implies that the pure BoNT seems unlikely to cause the food-borne poisoning, and conflicts with the previous description that the food-borne botulism is the most frequent among three botulism diseases [3].
To allow BoNT to reach it target a toxin complex (TC) is formed. The auxiliary nontoxic proteins in the complex play a role for the delivery of the toxin through the animal digestive system so that the botulinum TC exerts the oral toxicity. The Botulinum toxin complex is resistant to proteolytic digestion by pepsin and trypsin. "...The purified progenitor toxin of Clostridium botulinum type C strain 6814 (C‐6814) forms a large complex composed of 150‐kDa neurotoxin (NT), 130‐kDa nontoxic‐nonhemagglutinin (NTNHA), and hemagglutinin (HA) components. .."[8]
How the toxin complex enters the blood stream is still under debate. "...BoNTs use different strategies to pass through the intestinal barrier including passage of BoNT complexes containing hemagglutinins (HAs) via Microfold (M cells), HA-dependent perturbation of E-cadherin intercellular junctions between enterocytes and paracellular passage of BoNT complexes, and transcytosis of BoNT free of NAPs through certain intestinal epithelial cells..." [see reference 7]
See Selecte Abstracts at bottom of page for more information ---
HA3 Subcomponent of Botulinum Type C Progenitor Toxin
RCSB PDB: 2ZS6
"...The Clostridium botulinum type C 16S progenitor toxin contains a neurotoxin and several nontoxic components, designated nontoxic nonhemagglutinin (HA), HA1 (HA-33), HA2 (HA-17), HA3a (HA-22-23), and HA3b (HA-53). The HA3b subcomponent seems to play an important role cooperatively with HA1 in the internalization of the toxin by gastrointestinal epithelial cells via binding of these subcomponents to specific oligosaccharides. Nakamura et.al., investigated the sugar-binding specificity of the HA3b subcomponent using recombinant protein fused to glutathione S-transferase and determined the three-dimensional structure of the HA3a-HA3b complex based on X-ray crystallography..." Crystal Structure of the HA3 Subcomponent of Clostridium botulinum Type C Progenitor Toxin Nakamura, T., Kotani, M., Tonozuka, T., Ide, A., Oguma, K., Nishikawa, A. (2009) J.Mol.Biol. 385: 1193-1206. See also reference 10.
After BoNT enters the blood stream ot is transported to the neuromuscular junction. The receptor-binding domain provides cholinergic specificity and binds the toxin to the presynaptic receptors. The toxin then enters the neuronal cell via receptor-mediated endocytosis.
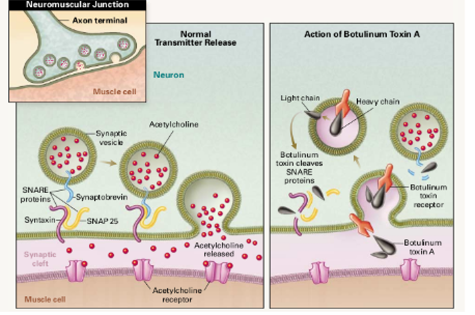
Mechanism of action at the neuromuscular junction
Mechanism of action of BoNT's leading to inhibition of neurotransmitter-containing vesicle release (right) compared to normal cell (left). Shown are BoNT binding to Botulinum toxin receptor, vesicle internatlizaton, translocation of the catalytic domain into the cytosol, and proteolitic cleavage on one of the SNARE proteins.
Image source: Rowland L.P. Stroke, spasticity, and botulinum toxin. N. Engl. J. Med. 2002;347:382–383.
Sensing the Deadliest Toxin: Technologies for Botulinum Neurotoxin Detection ref. 19-The light chain of BoNT is a zinc metalloprotease and is the active part of the toxin. It is translocated into the host cell cytoplasm where it cleaves the host protein SNAP-25, a member of the SNARE protein family which is responsible for fusion. The cleaved SNAP-25 is unable to mediate fusion of vesicles with the host cell membrane, thus preventing the release of the neurotransmitter acetylcholine from axon endings. This blockage is slowly reversed as the toxin loses activity and the SNARE proteins are slowly regenerated by the affected cell.
Role in disease
Botulinum toxin produced by Clostridium botulinum is the cause of botulism. Humans most commonly ingest the toxin from eating improperly-canned foods in which C. botulinum has grown. However, the toxin can also be introduced through an infected wound. In infants, the bacteria can sometimes grow in the intestines and produce botulinum toxin within the intestine and can cause a condition known as floppy baby syndrome. In all cases, the toxin can then spread, blocking nerves and muscle function. In severe cases, the toxin can block nerves controlling the respiratory system or heart, resulting in death. Botulism can be difficult to diagnose, as it may appear similar to diseases such as Guillain–Barré syndrome, myasthenia gravis, and stroke. Other tests, such as brain scan and spinal fluid examination, may help to rule out other causes. If the symptoms of botulism are diagnosed early, various treatments can be administered. In an effort to remove contaminated food which remains in the gut, enemas or induced vomiting may be used. Botulinum antitoxin is available and may be used to prevent the worsening of symptoms, though it will not reverse existing nerve damage.
Anti-toxins and mechanism to negate toxicity see:
Two preparations of botulinum antitoxins are available for treatment of botulism. Trivalent (A,B,E) botulinum antitoxin is derived from equine sources using whole antibodies. The second antitoxin is Heptavalent (A,B,C,D,E,F,G) botulinum antitoxin, which is derived from equine antibodies which have been altered to make them less immunogenic. This antitoxin is effective against all known strains of botulism
--Metals have been shown to ablate the action of BoNT. A screen of a series of metal salts showed marked inhibitory activity of group 11 and 12 metals against the BoNT/A light chain (LC) protease. Enzyme kinetics revealed that copper (I) and (II) cations displayed noncompetitive inhibition of the LC (Ki ≈ 1 μM), while mercury (II) cations were 10-fold more potent. [11]. In addition Crystallographic and mutagenesis studies elucidated a key binding interaction between Cys165 on BoNT/A LC and the inhibitory metals [11].https://pubs.acs.org/doi/10.1021/jacs.7b01084
--One natural compound, a nitrophenyl psoralen (NPP) was identified as a specific inhibitor of LCA with in vitro IC50 value of 4.74 ± 0.03 µM. NPP was able to rescue endogenous synaptosomal- associated protein-25 (SNAP-25) from the cleavage by BoNT/A in the human neuroblastoma cells with an IC50 of 12.2 ± 1.7 µM, as well as to prolong the time to block of neutrally elicited twitch tensions in isolated mouse phrenic nerve – hemidiaphragm preparations.
See review of article.
Selected Abstracts
Mauricio Montal, Botulinum Neurotoxin: A Marvel of Protein Design. Annual Review of Biochemistry Vol. 79:591-617 (2010)
"...Botulinum neurotoxin (BoNT), the causative agent of botulism, is acknowledged to be the most poisonous protein known. BoNT proteases disable synaptic vesicle exocytosis by cleaving their cytosolic SNARE (soluble NSF attachment protein receptor) substrates. BoNT is a modular nanomachine: an N-terminal Zn2+-metalloprotease, which cleaves the SNAREs; a central helical protein-conducting channel, which chaperones the protease across endosomes; and a C-terminal receptor-binding module, consisting of two subdomains that determine target specificity by binding to a ganglioside and a protein receptor on the cell surface and triggering endocytosis. For BoNT, functional complexity emerges from its modular design and the tight interplay between its component modules—a partnership with consequences that surpass the simple sum of the individual component's action. BoNTs exploit this design at each step of the intoxication process, thereby achieving an exquisite toxicity. This review summarizes current knowledge on the structure of individual modules and presents mechanistic insights into how this protein machine evolved to this level of sophistication. Understanding the design principles underpinning the function of such a dynamic modular protein remains a challenging task..."
Shenyan Gu, Sophie Rumpel, Jie Zhou, Jasmin Strotmeier, Hans Bigalke, Kay Perry,, Charles B. Shoemaker, Andreas Rummel, and Jin Rongsheng, (2012). Botulinum Neurotoxin Is Shielded by NTNHA in an Interlocked Complex. Science . 977-981.
Botulinum neurotoxins (BoNTs) are highly poisonous substances that are also effective medicines. Accidental BoNT poisoning often occurs through ingestion of Clostridium botulinum–contaminated food. Here, we present the crystal structure of a BoNT in complex with a clostridial nontoxic nonhemagglutinin (NTNHA) protein at 2.7 angstroms. Biochemical and functional studies show that NTNHA provides large and multivalent binding interfaces to protect BoNT from gastrointestinal degradation. Moreover, the structure highlights key residues in BoNT that regulate complex assembly in a pH-dependent manner. Collectively, our findings define the molecular mechanisms by which NTNHA shields BoNT in the hostile gastrointestinal environment and releases it upon entry into the circulation. These results will assist in the design of small molecules for inhibiting oral BoNT intoxication and of delivery vehicles for oral administration of biologics.
Montecucco C1, Schiavo G. (1995) Structure and function of tetanus and botulinum neurotoxins. Annual Review of Biochemistry. Nov;28(4):423-72.
Tetanus and botulinum neurotoxins are produced by Clostridia and cause the neuroparalytic syndromes of tetanus and botulism. Tetanus neurotoxin acts mainly at the CNS synapse, while the seven botulinum neurotoxins act peripherally. Clostridial neurotoxins share a similar mechanism of cell intoxication: they block the release of neurotransmitters. They are composed of two disulfide-linked polypeptide chains. The larger subunit is responsible for neurospecific binding and cell penetration. Reduction releases the smaller chain in the neuronal cytosol, where it displays its zinc-endopeptidase activity specific for protein components of the neuroexocytosis apparatus. Tetanus neurotoxin and botulinum neurotoxins B, D, F and G recognize specifically VAMP/ synaptobrevin. This integral protein of the synaptic vesicle membrane is cleaved at single peptide bonds, which differ for each neurotoxin. Botulinum A, and E neurotoxins recognize and cleave specifically SNAP-25, a protein of the presynaptic membrane, at two different sites within the carboxyl-terminus. Botulinum neurotoxin type C cleaves syntaxin, another protein of the nerve plasmalemma. These results indicate that VAMP, SNAP-25 and syntaxin play a central role in neuroexocytosis. These three proteins are conserved from yeast to humans and are essential in a variety of docking and fusion events in every cell. Tetanus and botulinum neurotoxins form a new group of zinc-endopeptidases with characteristic sequence, mode of zinc coordination, mechanism of activation and target recognition. They will be of great value in the unraveling of the mechanisms of exocytosis and endocytosis, as they are in the clinical treatment of dystonias.
Kwangkook Lee , Shenyan Gu , Lei Jin, Thi Tuc Nghi Le, Luisa W. Cheng, Jasmin Strotmeier, Anna Magdalena Kruel, Guorui Yao, Kay Perry, Andreas Rummel , Rongsheng Jin. (2013) Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLOS Pathogens. https://doi.org/10.1371/journal.ppat.1003690
Botulinum neurotoxins (BoNTs) are produced by Clostridium botulinum and cause the fatal disease botulism, a flaccid paralysis of the muscle. BoNTs are released together with several auxiliary proteins as progenitor toxin complexes (PTCs) to become highly potent oral poisons. Here, we report the structure of a ∼760 kDa 14-subunit large PTC of serotype A (L-PTC/A) and reveal insight into its absorption mechanism. Using a combination of X-ray crystallography, electron microscopy, and functional studies, we found that L-PTC/A consists of two structurally and functionally independent sub-complexes. A hetero-dimeric 290 kDa complex protects BoNT, while a hetero-dodecameric 470 kDa complex facilitates its absorption in the harsh environment of the gastrointestinal tract. BoNT absorption is mediated by nine glycan-binding sites on the dodecameric sub-complex that forms multivalent interactions with carbohydrate receptors on intestinal epithelial cells. We identified monosaccharides that blocked oral BoNT intoxication in mice, which suggests a new strategy for the development of preventive countermeasures for BoNTs based on carbohydrate receptor mimicry.
Readings and References:
1- P K Nigam and Anjana Nigam. Botulinum Toxin. Indian J Dermatol. 2010 Jan-Mar; 55(1): 8–14. doi: [10.4103/0019-5154.60343]
2-Sellin LC. The pharmacological mechanism of botulism. Trends Pharmacol Sci. 1985;6:80–2.
3-Yoshimasa Sagane, Ken Inui, Shin-Ichiro Miyashita, Keita Miyata, Tomonori Suzuki, Koichi Niwa and Toshihiro Watanabe. (2012) Botulinum Toxin Complex: A Delivery Vehicle of Botulinum Neurotoxin Traveling Digestive Tract. DOI: 10.5772/46023.
4- Nipawan Nuemket,a Yoshikazu Tanaka,b,c,* Kentaro Tsukamoto,d Takao Tsuji,d Keiji Nakamura,e Shunji Kozaki,e Min Yao,a,e and Isao Tanakaa,c,*Preliminary X-ray crystallographic study of the receptor-binding domain of the D/C mosaic neurotoxin from Clostridium botulinum . Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 66(Pt 5): 608–610. doi: [10.1107/S1744309110012182]
5-Amornrat Aroonnual, Tavan Janvilisri, Surang Chankhamhaengdecha (2016) Botolinum toxins: their structure, properties, and genetics Journal of Medicine and Health (2016)
6-Lebeda et. al. (2008), Onset dynamics of type A botulinum neurotoxin-induced paralysis.
7-Fujinaga, Yukako & Popoff, Michel. (2017). Translocation and dissemination of botulinum neurotoxin from the intestinal tract. Toxicon. 147.
8-Hirokazu Kouguchi Toshihiro Watanabe Yoshimasa Sagane Tohru Ohyama (2001) Characterization and reconstitution of functional hemagglutinin of the Clostridium botulinum type C progenitor toxin. FEBS Press. December 2001.
9- HA3 subcomponent of botulinum type C progenitor toxin DOI: 10.2210/pdb2ZS6/pdb
10-Kwok-Ho Lam and Rongsheng Jin*. Architecture of the botulinum neurotoxin complex: a molecular machine for protection and delivery. (2015) Curr Opin Struct Biol. 2015 Apr; 31: 89–95. doi: [10.1016/j.sbi.2015.03.013]
11-Paul T. Bremer, Sabine Pellett, James P. Carolan, William H. Tepp, Lisa M. Eubank, Karen N. Allen , Eric A. Johnson, and Kim D. Janda (2017) Metal Ions Effectively Ablate the Action of Botulinum Neurotoxin A. J. Am. Chem. Soc., 2017, 139 (21), pp 7264–7272. DOI: 10.1021/jacs.7b01084