Anandamine Molecule - The bliss molecule
Ball and Stick Model for Anandamide Molecule
To View the Anandamide Molecule in 3D --->>in 3D with Jsmol
Overview
Anandamide N-arachidonoylethanolamine or AEA)-- C₂₂H₃₇NO₂-- is a fatty acid amide molecule (endocannabinoid) which acts as a neurotransmitter, and has a structure very similar to that of tetrahydrocannabinol (THC) the active constituent of cannabis. Endocannabinoids and their receptors are found throughout the body: in the brain, lungs, digestive system, connective tissues, hormone releasing glands, skin/hair, bone, the immune system, and the reproductive organs. Anandamide was first described (and named) in 1992 by Raphael Mechoulam and his lab members W. A. Devane and Lumír Hanuš.
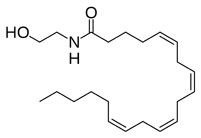
Molecular Structure of Ananadamide
It is derived from the non-oxidative metabolism of eicosatetraenoic acid (arachidonic acid), an ω-6 polyunsaturated fatty acid (Note: arachidonic acid is not one of the essential fatty acids. However, it does become essential if there is a deficiency in linoleic acid or if there is an inability to convert linoleic acid to arachidonic acid.
Anandamide is the first endogenous ligand of central cannabinoid receptors (CB1 receptor), that was isolated from porcine brain in 1992 (1). It's effects can occur in either the central or peripheral nervous system, CB1 receptors are found in the central nervous system, and CB2 cannabinoid receptors in the periphery, the latter are mainly involved in functions of the immune system. (2). Anandamide is often called the "bliss molecule" -- the name anandamine is taken from the Sanskrit word anana which means "joy, bliss or delight.
Cannabinoid receptors were originally discovered as being sensitive to Δ9-tetrahydrocannabinol (Δ9-THC, commonly called THC), which is the primary psychoactive cannabinoid found in cannabis. The discovery of anandamide came from research into CB1 and CB2, as it was inevitable that a naturally occurring (endogenous) chemical would be found to affect these receptors. Since its discovery, several CB1-mediated effects have been reported for this endogenous cannabinoid in numerous mammalian tissues (see ref. 3,4).
Biosythesis and degradation
Anandamiide is synthesized and released “on demand” by precursors located in neuron cell membranes in the brain in response to a rise in intracellular calcium (5a). There are several pathways now know for synthesis of anandamine. Anandamide is primarily formed together with other N-acylethanolamines (NAEs) in a two-step pathway, which is composed of Ca2+-dependent N-acyltransferase and N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). (5b)
Primary synthesis of anandamine
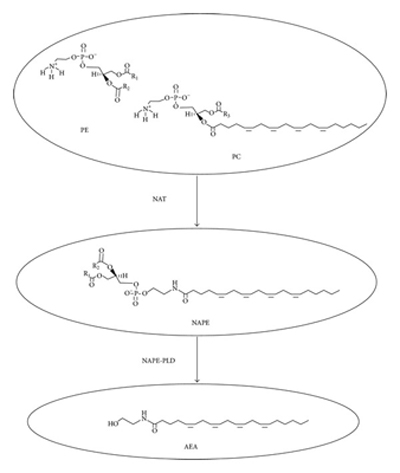
Synthesis of N-arachidonoylethanolamine (AEA). N-acyltransferase (NAT) catalyzes the transfer of arachidonic acid (AA) from phosphatidylcholine (PC) to phosphatidylethanolamine (PE) to form N-arachidonoyl-phosphatidylethanolamine (NAPE). NAPE is then converted into AEA in a one-step hydrolysis reaction catalyzed by the NAPE-specific phospholipase D (NAPE-PLD) (source: reference 7)
NAPE itself is made by the bonding of arachidonic acid (an omega-6 EFA) and a free amine through the action of the enzyme N-acyltransferase. Variations in dietary consumption of arachidonic acid can alter the levels of anandamide present in the brain. (6)
Endogenous anandamide is present at very low levels and has a very short half-life due to the action of the enzyme fatty acid amide hydrolase (FAAH), which breaks it down into free arachidonic acid and ethanolamine. Studies of piglets show that dietary levels of arachidonic acid and other essential fatty acids affect the levels of anandamide and other endocannabinoids in the brain. High fat diet feeding in mice increases levels of anandamide in the liver and increases lipogenesis. This suggests that anandamide may play a role in the development of obesity, at least in rodents. (source -- Wikipedia)
Effects of Anandamide
Anandamide's effects can occur in either the central or peripheral nervous system. These distinct effects are mediated primarily by CB1 cannabinoid receptors in the central nervous system, and CB2 cannabinoid receptors in the periphery. (2) The latter are mainly involved in functions of the immune system. Anandamide has many effects in the body, including appetite regulation, reward, cell regulation, memory, pain relief, and mood. Because CB1 and CB2 receptors are so widespread, it is possible that anandamide has more effects on the body that have not yet been discovered.
Pain and inflammation
A large body of evidence now exists to substantiate that the endocannabinoid, anandamide, activates TRPV1 receptors.The activation of TRPV1 receptors by anandamide has potential implications in the treatment of inflammatory, respiratory and cardiovascular disorders. The relative importance of anandamide as a physiological and/or pathophysiological TRPV1 receptor agonist in comparison to other potential candidates has yet to be revealed. (11)
Early stage embryo
Anandamide is also important for implantation of the early stage embryo in its blastocyst form into the uterus. Therefore, cannabinoids such as Δ9-THC might influence processes during the earliest stages of human pregnancy.
Exercise and Anandamine
It has been shown that aerobic exercise causes an increase in plasma anandamide levels, where the magnitude of this increase is highest at moderate exercise intensity ( i.e., exercising at ~70–80% maximum heart rate). Increases in plasma anandamide levels are associated with psychoactive effects because anandamide is able to cross the blood–brain barrier and act within the central nervous system. (8) Thus, because anandamide is a euphoriant and aerobic exercise is associated with euphoric effects, it has been proposed that anandamide partly mediates the short-term mood-lifting effects of exercise (e.g., the euphoria of a runner's high) via exercise-induced increases in its synthesis (8).
Chocolate contains a compound that prevents breakdown of amandamine
Compounds, such as N-acylethanolamines, that prevent the breakdown of anandamide have also been found in chocolate. Perhaps part of the pleasure we get from eating chocolate comes from the effect that the anandamide and N-acylethanolamines it contains has upon our brains. (9)
Lymphocyte and macrophage function
Anandamide and 2-arachidonoyl-glycerol, another putative “endogenous cannabinoid”, have been shown to affect lymphocyte and macrophage function (12), even though it is not clear yet whether these immunomodulatory actions are mediated by the CB1 or the “peripheral” CB2 cannabinoid receptor subtype.
Amandamide and Cancer
It was suggested that AEA might inhibit tumor cell proliferation or induce apoptosis independently of CB1 and CB2 receptors, via interaction with the type 1 vanilloid receptor (VR1). VR1 is an ion channel expressed almost exclusively by sensory neurons, activated by pH, noxious heat (> 48 degree centigrade) and plant toxins and is thought to play an important role in nociception. Cervical cancer cells are sensitive to AEA-induced apoptosis via VR1 that is aberrantly expressed in vitro and in vivo while CB1 and CB2 receptors play a protective role. (PMID: 15047233). Novel prostaglandins (prostaglandin glycerol esters and prostaglandin ethanolamides) are COX-2 oxidative metabolites of endogenous cannabinoids (such as Anandamide). Recent evidence suggests that these new types of prostaglandins are likely novel signaling mediators involved in synaptic transmission and plasticity. (PMID: 16957004).
Anandamide dose-dependently inhibited the proliferation of MCF-7 and EFM-19 cells--in vitro-- with IC50 values between 0.5 and 1.5 μM and 83–92% maximal inhibition at 5–10 μM. The proliferation of several other nonmammary tumoral cell lines was not affected by 10 μM anandamide. This data suggest that anandamide blocks human breast cancer cell proliferation through CB1-like receptor-mediated inhibition of endogenous prolactin action at the level of prolactin receptor.(10)
REFERENCES
- Devane, W.; Hanus, L; Breuer, A; Pertwee, R.; Stevenson, L.; Griffin, G; Gibson, D; Mandelbaum, A; Etinger, A; Mechoulam, R (18 December 1992). "Isolation and structure of a brain constituent that binds to the cannabinoid receptor". Science. 258 (5090): 1946–1949.
- Pacher P, Batkai S, Kunos G (2006). "The Endocannabinoid System as an Emerging Target of Pharmacotherapy". Pharmacol. Rev. 58 (3): 389–462
- Mechoulam R, Fride E (1995). "The unpaved road to the endogenous brain cannabinoid ligands, the anandamides". In Pertwee RG. Cannabinoid receptors. Boston: Academic Press. pp. 233–258.
- Wang, J.; Ueda, N. (2009). "Biology of endocannabinoid synthesis system". Prostaglandins & Other Lipid Mediators. 89 (3–4): 112–119.
- 5a- Endocannabinoids: Anandamide, 2‑Arachidonoylglycerol and Related Lipids, (5b) Jie Liu, Lei Wang, Judith Harvey-White, Douglas Osei-Hyiaman, Raj Razdan, Qian Gong, Andrew C. Chan, Zhifeng Zhou, Bill X. Huang, Hee-Yong Kim, and George Kuno (2006) A biosynthetic pathway for anandamide. PNAS 103 (36) 13345-13350;
- Cannabinoid Science 101: What is Anandamide?
- Thangesweran Ayakannu, Anthony H. Taylor, Timothy H. Marczylo, Jonathon M. Willets, and Justin C. Konje (2013)The Endocannabinoid System and Sex Steroid Hormone-Dependent Cancers. Int J Endocrinol. 2013; 2013: 259676.
- Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V, Maccarrone M (2014). "Physical activity and the endocannabinoid system: an overview". Cell. Mol. Life Sci. 71(14): 2681–2698.
- Anandamide --The molecule of extreme pleasure. Sujit Kumar Kar, S.K. Foundation, Orissa, India .
- Luciano De Petrocellis, Dominique Melck, Antonella Palmisano, Tiziana Bisogno, Chiara Laezza, Maurizio Bifulco, and Vincenzo Di Marzo. (1998) The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. PNAS 1998 Jul 7; 95(14): 8375–8380.
- Ruth A Ross, (2003) Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003 Nov; 140(5): 790–801.
- J. Neuroimmunology. (2007) Anandamide and Δ9-Tetrahydrocannabinol Directly Inhibit Cells of the Immune System via CB2 Receptors