urons that have those receptors.
Pseudoephedrine is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It is used as a nasal/sinus decongestant and stimulant, or as a wakefulness-promoting agent. The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in many over-the-counter preparations either as a single ingredient or, more commonly, in combination with antihistamines, guaifenesin, dextromethorphan, paracetamol (acetaminophen), and/or NSAIDs (e.g., aspirin, ibuprofen, etc.).
Chemistry
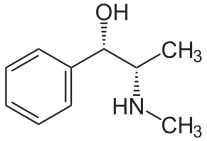 |
Pseudoephedrine is a diastereomer of ephedrine and is readily reduced into methamphetamine or oxidized into methcathinone. |
Pseudoephedrine is a diastereomer of ephedrine and is readily reduced into methamphetamine or oxidized into methcathinone.
Nomenclature
The dextrorotary (+)- or d- enantiomer is (1S,2S)-Pseudoephedrine, whereas the levorotating (−)- or l- form is (1R,2R)-Pseudoephedrine.
In the outdated d/l system (+)-Pseudoephedrine is also referred to as l-Pseudoephedrine and (−)-Pseudoephedrine as d-Pseudoephedrine (in the Fisher projection then the phenylring is drawn at bottom). [2] [3]
Often the d/l system (with small caps) and the d/l system (with lower-case) are confused. The result is that the dextrorotary d-Pseudoephedrine is wrongly named d-Pseudoephedrine and the levorotary l-Ephedrine (the diastereomer) wrongly l-Ephedrine.
The IUPAC names of the two enantiomers are (1S,2S)- respectively (1R,2R)-2-methylamino-1-phenylpropan-1-ol. Synonyms for both are psi-Ephedrine and threo-Ephedrine.
Pseudoephedrine is the International Nonproprietary Name (INN) of the (+)-form, when used as pharmaceutical substance. [4]
Synthesis
Although pseudoephedrine occurs naturally as an alkaloid in certain plant species (for example, as a constituent of extracts from the ephedra species, also known as Ma Huang, in which it occurs together with other isomers of ephedrine), the majority of pseudoephedrine produced for commercial use is derived from yeast fermentation of dextrose in the presence of benzaldehyde. In this process, specialized strains of yeast (typically a variety of Candida utilis or Saccharomyces cerevisiae) are added to large vats containing water, dextrose and the enzyme pyruvate decarboxylase (such as found in beets and other plants). After the yeast has begun fermenting the dextrose, the benzaldehyde is added to the vats, and in this environment the yeast converts the ingredients to the precursor l-phenylacetylcarbinol (L-PAC). L-PAC is then chemically converted to pseudoephedrine via reductive amination.[5]
The bulk of pseudoephedrine is produced by commercial pharmaceutical manufacturers in India and China, where economic and industrial conditions favor the mass production of pseudoephedrine for export.[6]
Mechanism of action
Pseudoephedrine is a sympathomimetic amine. Its principal mechanism of action relies on its indirect action on the adrenergic receptor system. The vasoconstriction that pseudoephedrine produces is believed to be principally an adrenergic receptor response. [7]
While it may have weak or no direct agonist activity at α- and β-adrenergic receptors, the principal mechanism is to cause the release of endogenous norepinephrine (noradrenaline) from storage vesicles in presynaptic neurons. The displaced noradrenaline is released into the neuronal synapse where it is free to activate the postsynaptic adrenergic receptors. These adrenergic receptors are located on the muscles lining the walls of blood vessels . When these receptors are activated by noradrenaline, the muscles contract, causing the blood vessels to constrict (vasoconstriction). The constricted blood vessels now allow less fluid to leave the blood vessels and enter the nose, throat and sinus linings, which results in decreased inflammation of nasal membranes as well as decreased mucus production. Thus, by constriction of blood vessels, mainly those located in the nasal passages, pseudoephedrine causes a decrease in the symptoms of nasal congestion.
Medical uses
Pseudoephedrine is a stimulant, but it is well known for shrinking swollen nasal mucous membranes; for this reason, it is often used as a decongestant. It reduces tissue hyperemia, edema, and nasal congestion commonly associated with colds or allergies . Other beneficial effects may include increasing the drainage of sinus secretions, and opening of obstructed Eustachian tubes. The same vasoconstriction action can also result in hypertension, which is a noted side effect of pseudoephedrine.
Pseudoephedrine can be used either as oral or as topical decongestant. The advantage of oral pseudoephedrine over topical nasal preparations, such as oxymetazoline, is that it does not cause rebound congestion (rhinitis medicamentosa). However, due to its stimulating qualities , it is more likely to cause adverse effects, including hypertension, sweating, insomnia, and anxiety.
Pseudoephedrine may be useful as antitussive drug (suppressing of cough). [8]
Indications
Pseudoephedrine is indicated for the treatment of:
- nasal congestion
- sinus congestion
- Eustachian tube congestion.[9]
Pseudoephedrine is also indicated for vasomotor rhinitis, and as an adjunct to other agents in the optimum treatment of allergic rhinitis, croup, sinusitis, otitis media, and tracheobronchitis.[9]
Pseudoephedrine is also used as first-line therapy of priapism. Erection is largely a parasympathetic response, so the sympathetic action of pseudoephedrine may serve to relieve this condition.
Treatment for urinary incontinence is an off-label use (a.k.a. "unlabeled use") for these medications .[10]
Adverse effects
Common adverse drug reactions (ADRs) associated with pseudoephedrine therapy include: CNS stimulation, insomnia, nervousness, excitability, dizziness and anxiety. Infrequent ADRs include: tachycardia and/or palpitations. Rarely, pseudoephedrine therapy may be associated with mydriasis (blurred vision), hallucinations, arrhythmias, hypertension, seizures and ischemic colitis;[11] as well as severe skin reactions known as recurrent pseudo-scarlatina, systemic contact dermatitis, and nonpigmenting fixed drug eruption.[12] Pseudoephedrine, particularly in high doses , may also cause episodes of paranoid psychosis.[13] It has also been reported that pseudoephedrine, amongst other sympathomimetic agents, may be associated with the occurrence of stroke.[14]
Precautions and contraindications
It is recommended that pseudoephedrine not be used in patients with: diabetes mellitus, cardiovascular disease, severe or uncontrolled hypertension, severe coronary artery disease, prostatic hypertrophy, hyperthyroidism, closed angle glaucoma, or pregnant women.[11] Patients who are prone to anxiety or panic attacks should use pseudoephedrine with caution, as anxiety and restlessness are common side effects, mostly due to the drug's stimulant properties. Since nasal congestion is considered to be a relatively minor ailment, alternatives are preferred in patients with these conditions. Appropriate alternatives may include saline sprays/instillations, depending on the patient 's condition. Topical decongestants should be used with caution and for no longer than three days to avoid Rhinitis medicamentosa.People with bipolar disorder should use care when taking pseudoephedrine, as it can cause insomnia and thus trigger a manic episode.
Interactions
Concomitant or recent (previous fourteen days) monoamine oxidase inhibitor (MAOI) use can lead to hypertensive reactions, including hypertensive crises.[11]
The antihypertensive effects of methyldopa, mecamylamine, reserpine and veratrum alkaloids may be reduced by sympathomimetics. Beta-adrenergic antagonists may also interact with sympathomimetics. Increase of ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Antacids increase the rate of pseudoephedrine absorption, kaolin decreases it.
Common brand names
Sudafed is a trademark for a common brand that contains pseudoephedrine, although Sudafed PE does not contain it.
Sports
Pseudoephedrine was on the banned substances IOC list until 2004, when the WADA list replaced the IOC list. Although WADA initially only monitored pseudoephedrine, it is once again on the banned list starting January 1, 2010.[17]
Detection of use
Pseudoephedrine may be quantitated in blood, plasma or urine to monitor any possible performance-enhancing use by athletes, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Many commercial immunoassay screening tests directed at the amphetamines cross-react appreciably with pseudoephedrine, but chromatographic techniques can easily distinguish pseudoephedrine from other phenethylamine derivatives. Blood or plasma pseudoephedrine concentrations are typically in the 50-300 µg/L range in persons taking the drug therapeutically, 500-3000 µg/L in abusers or poisoned patients and 10–70 mg/L in cases of acute fatal overdosage.[20][21]
United States
The United States Congress has recognized that pseudoephedrine is used in the illegal manufacture of methamphetamine. In 2005, the Committee on Education and the Workforce heard testimony concerning education programs and state legislation designed to curb this illegal practice.
Pseudoephedrine was defined as a "scheduled listed chemical product" under 21 U.S.C. § 802(45(A)). The act included the following requirements for merchants ("regulated sellers") who sell such products:
- Required verification of proof of identity of all purchasers
- Required protection and disclosure methods in the collection of personal information
- Required reports to the Attorney General of any suspicious payments or disappearances of the regulated products
- Required training of employees with regard to the requirements of the CMEA. Retailers must self-certify as to training and compliance.
- The non-liquid dose form of regulated products may only be sold in unit dose blister packs
- Regulated products must be stored behind the counter or in a locked cabinet in such a way as to restrict public access
- Sales limits (per customer):
- Daily sales limit must not exceed 3.6 grams of pseudoephedrine base without regard to the number of transactions
- 30-day (not monthly) sales limit ”must not exceed 7.5 grams of pseudoephedrine base if sold by mail order or "mobile retail vendor"
- 30-day purchase limit must not exceed 9 grams of pseudoephedrine base. (A misdemeanor possession offense under 21 U.S.C. § 844a for the person who buys it.)
In regards to the identification that may be used by an individual buying pseudoephedrine products the following constitute acceptable forms of identification:
- US passport
- Alien registration or permanent resident card
- Unexpired foreign passport with temporary I-551 stamp
- Unexpired Employment Authorization Document
- Driver's License or Government issued identification card (including Canadian driver's license)
- School ID with picture
- Voter's Registration card
- US Military Card
- Native American tribal documents
References
- Laurence L Brunton, ed (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill Medical Publishing Division.
- Popat N. Patil, A. Tye and J.B. LaPidus A pharmacological study of the ephedrine isomers JPET May 1965 vol. 148, no. 2, pp. 158-168. PDF
- Martindale (1989). Edited by Reynolds JEF. ed. Martindale: The complete drug reference (29th ed.). London: Pharmaceutical Press.
- Proposed International Non-Proprietary Names (Prop. I.N.N.): List 11 WHO Chronicle, Vol. 15, No. 8, August 1961, pp. 314-320
- Oliver AL, Anderson BN, Roddick FA (1999). "Factors affecting the production of L-phenylacetylcarbinol by yeast: a case study". Adv. Microb. Physiol.. Advances in Microbial Physiology 41: 1-45.
- Suo, Steve. Clamp down on shipments of raw ingredients. The Oregonian; 6 October 2004. From a version reprinted on a U.S. congressional caucus website.
- Drew, et al. Comparison of the effects of D-(-)-ephedrine and L-(+)-pseudoephedrine on the cardiovascular and respiratory systems in man. Br J Clin Pharmacol. 1978; 6, p 225; PDF
- Kiyoshi Minamizawa, et al. Effect of d-Pseudoephedrine on Cough Reflex and Its Mode of Action in Guinea PigsJournal of Pharmacological Sciences, Vol. 102 (2006), No. 1 pp.136-142. PDF
- Bicopoulos D, editor. AusDI: Drug information for the healthcare professional, 2nd edition. Castle Hill: Pharmaceutical Care Information Services; 2002.
- http://www.aafp.org/afp/2005/0115/p315.html
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- Vidal C, Prieto A, Pérez-Carral C, Armisén M (April 1998). "Nonpigmenting fixed drug eruption due to pseudoephedrine". Ann. Allergy Asthma Immunol. 80 (4): 309-10. d
- Adco-Tussend
- Cantu C, Arauz A, Murillo-Bonilla LM, López M, Barinagarrementeria F (July 2003). "Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs". Stroke 34 (7): 1667-72. doi:10.1161/01.STR.0000075293.45936.FA. PMID 12791938.
- Hatton RC, Winterstein AG, McKelvey RP, Shuster J, Hendeles L (March 2007). "Efficacy and safety of oral phenylephrine: systematic review and meta-analysis". Ann Pharmacother 41 (3): 381–90. doi:10.1345/aph.1H679
- Microsoft Word - RedListE2007.doc
- http://www.wada-ama.org/en/News-Center/Articles/WADA-2010-Prohibited-List-Now-Published/
- http://assets.espn.go.com/oly/summer00/news/2000/0925/776388.html
- http://web.archive.org/web/20010715112418/http://www.intlgymnast.com/news2000/oct3.html
- Boland DM, Rein J, Lew EO, Hearn WL. Fatal cold medication intoxication in an infant. J. Anal. Toxicol. 27: 523-526, 2003.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1344-1346.
- http://www.projectstop.com.au/
- "Ephedrine and Pseudoephedrine to Become Controlled Drugs"
- "Chemical Brothers", Listener
- Digest No. 1776
- "Drug Safety Update, March 2008" (pdf). The Medicines and Healthcare products Regulatory Agency and the Commission on Human Medicines. 2008-06-30.
- http://www.oregonlive.com/special/oregonian/meth/stories/index.ssf?/oregonian/meth/1004_lobbyistsandloopholes.html
