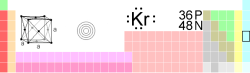
|
|||||
| General | |||||
|---|---|---|---|---|---|
| Name, Symbol, Number | krypton, Kr, 36 | ||||
| Chemical series | Noble gases | ||||
| Group, Period, Block | 18 (VIIIA), 4 , p | ||||
| Density, Hardness | 3.708 kg/m3 (273 K), NA | ||||
| Appearance | colorless |
||||
| Atomic properties | |||||
| Atomic weight | 83.798 amu | ||||
| Atomic radius (calc.) | no data (88) pm | ||||
| Covalent radius | 110 pm | ||||
| van der Waals radius | 202 pm | ||||
| Electron configuration | [Ar]3d10 4s2 4p6 | ||||
| e- 's per energy level | 2, 8, 18, 8 | ||||
| Oxidation states (Oxide) | 0 (unknown) | ||||
| Crystal structure | cubic face centered | ||||
| Physical properties | |||||
| State of matter | gas (nonmagnetic) | ||||
| Melting point | 115.79 K (-251.25 °F) | ||||
| Boiling point | 119.93 K (-153.22 °F) | ||||
| Molar volume | 27.99 ×10-6 m3/mol | ||||
| Heat of vaporization | 9 .029 kJ/mol | ||||
| Heat of fusion | 1 .638 kJ/mol | ||||
| Vapor pressure | NA | ||||
| Speed of sound | 1120 m/s at 293.15 K | ||||
| Miscellaneous | |||||
| Electronegativity | 3.00 (Pauling scale) | ||||
| Specific heat capacity | 248 J/(kg*K) | ||||
| Electrical conductivity | no data | ||||
| Thermal conductivity | 0.00949 W/(m*K) | ||||
| 1st ionization potential | 1350.8 kJ/mol | ||||
| 2nd ionization potential | 2350.4 kJ/mol | ||||
| 3rd ionization potential | 3565 kJ/mol | ||||
| 4th ionization potential | 5070 kJ/mol | ||||
| 5th ionization potential | 6240 kJ/mol | ||||
| 6th ionization potential | 7570 kJ/mol | ||||
| 7th ionization potential | 10710 kJ/mol | ||||
| 8th ionization potential | 12138 kJ/mol | ||||
| SI units & STP are used except where noted. | |||||
Krypton is a chemical element in the periodic table that has the symbol Kr and atomic number 36. A colorless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionating liquefied air, and is often used with other rare gases in fluorescent lamps. Krypton is inert for most practical purposes but it is known to form compounds with fluorine. Krypton can also form clathrates with water when atoms of it are trapped in a lattice of the water molecules.
Krypton, a so-called noble gas due to its very low chemical reactivity, is characterized by a brilliant green and orange spectral signature. It is one of the products of uranium fission. Solidified krypton is white and crystalline with a face-centered cubic crystal structure which is a common property of all "rare gases."
Applications
The SI standard definition of the length of the metre was, from 1960 to 1983, based on the light emitted by excited krypton atoms: specifically, the metre was defined as 1,650,763.73 wavelengths of the orange-red emission line emitted by krypton-86 atoms.
Krypton clathrates have been made with hydroquinone and phenol. Kr-85 is used in chemical analysis. When it is placed in various solids kryptonates are formed and their activity is sensitive to surface chemical reactions. This noble gas is also used in photographic flash lamps needed for high-speed photography but this use is limited because of the high cost of krypton.
History
Krypton (Greek kryptos meaning "hidden") was discovered in 1898 by William Ramsay and Morris Travers in residue left from evaporating nearly all components of liquid air. In 1960 an international agreement defined the metre in terms of light emitted from a krypton isotope. This agreement replaced the longstanding standard metre located in Paris which was a metal bar made of a platinum-iridium alloy (the bar was originally estimated to be one ten millionth of a quadrant of the earth's polar circumference). In October 1983 the krypton standard was in turn replaced by the Bureau International des Poids et Mesures (International Bureau of Weights and Measures). A metre is now defined as the distance that light travels in a vacuum during 1/299,792,458 s.
Occurrence
The concentration of this gas in earth's atmosphere is about 1 ppm. It can be extracted from liquid air by fractional distillation.
Compounds
Like the other rare gases krypton is widely considered to be chemically inert. However, studies conducted since the 1960s have uncovered some compounds of krypton. Krypton difluoride has been made in gram quantities and can be produced in several different ways. Other fluorides and a salt of a krypton called oxyacid have also been found. ArKr+ and KrH+ molecule-ions have been investigated and there is evidence for KrXe or KrXe+.
Isotopes
Naturally occurring krypton is composed of six stable isotopes. Krypton's spectral signature is easily produced with some very sharp lines. Kr-81 is the product of atmospheric reactions with the other naturally occurring isotopes of krypton. It is radioactive with a half-life of 250,000 years. Like xenon, krypton is highly volatile when it is near surface waters and Kr-81 has therefore been used for dating old (50,000 - 800,000 year) groundwater. Kr-85 is an inert radioactive noble gas with a half-life of 10.76 years, that is produced by fission of uranium and plutonium. Sources have included nuclear bomb testing, nuclear reactors, and the release of Kr-85 during the reprocessing of fuel rods from nuclear reactors. A strong gradient exists between the northern and southern hemispheres where concentrations at the North Pole are approximately 30% higher than the South Pole due to convective mixing.
External links
- EnvironmentalChemistry.com – Krypton (http://environmentalchemistry.com/yogi/periodic/Kr.html)