Sucrose Molecule
Sucrose (C12H22O11) is the chemical name of table sugar. Sucrose is a disaccharide; each molecule consists of two "simple" sugars (a glucose and a fructose), called monosaccharides.
Composition
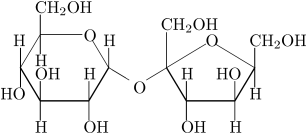
The sucrose molecule is a disaccharide composed of one molecule of glucose connected via an glycosidic bond to one molecule of fructose.
To View the Sucrose Molecule in 3D --->>in 3D with Jmol
Chemical and Physical Properties of Sucrose
In sucrose, the components glucose and fructose are linked via an acetal bond between C1 on the glucosyl subunit and C2 on the fructosyl unit. The bond is called a glycosidic linkage. Glucose exists predominantly as two isomeric "pyranoses" (α and β), but only one of these forms links to the fructose. Fructose itself exists as a mixture of "furanoses", each of which having α and β isomers, but only one particular isomer links to the glucosyl unit. What is notable about sucrose is that, unlike most disaccharides, the glycosidic bond is formed between the reducing ends of both glucose and fructose, and not between the reducing end of one and the nonreducing end of the other. This linkage inhibits further bonding to other saccharide units. Since it contains no anomeric hydroxyl groups, it is classified as a non-reducing sugar.
Sucrose crystallizes in the monoclinic space group P21 with room-temperature lattice parameters a = 1.08631 nm, b = 0.87044 nm, c = 0.77624 nm, β = 102.938°.
The purity of sucrose is measured by polarimetry, through the rotation of plane-polarized light by a solution of sugar. The specific rotation at 20 °C using yellow "sodium-D" light (589 nm) is +66.47°. Commercial samples of sugar are assayed using this parameter. Sucrose does not deteriorate at ambient conditions.
Production
Sucrose is generally extracted from sugar cane or sugar beet and then purified and crystallized. Other (minor) commercial sources are sorghum and sugar maples.
Usage
Pure sucrose is the most common sweetener in the modern, industrialized world. People, and in fact most other mammals except members of the cat family, will gladly accept a food sweetened with sucrose, even if they aren't hungry. Processed food and junk food often have sucrose added.
Health effects
Sucrose has several adverse health effects. The most common is tooth decay, in which bacteria in the mouth turn sucrose into acid that attacks tooth enamel. Sucrose has a high calorie content and is also believed to cause obesity. People with diabetes mellitus need to control their intake of sucrose.
Sugar substitutes
Because of the health effects of sucrose, several substitutes have been developed, although none appear to be as versatile as sugar in cooking and they may have other health consequences.