GABA Molecule
The GABA (Gamma-aminobutyric acid) molecule is the nucleotide known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. ATP also plays an important role in the synthesis of nucleic acids.
For 3-D Structure of this image using JsmolClick
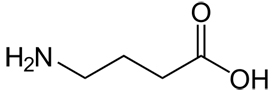
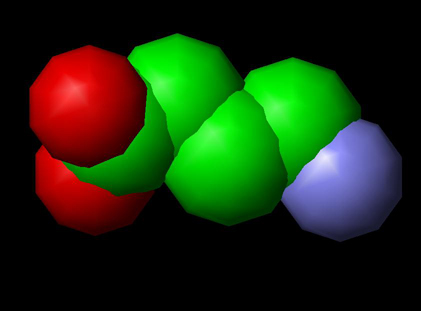
Image: Molecular Structure of GABA
Gamma-aminobutyric acid (usually abbreviated to GABA) with Chemical Formula C4H9NO2, and Molar mass 103.12 g/mol is an inhibitory neurotransmitter found in the nervous systems of widely-divergent species. It is the chief inhibitory neurotransmitter in the central nervous system and also in the retina. GABA is an amino acid, but is not found in proteins. Although some GABA can be found in pancreatic islet cells and kidney, there are no significant amounts of GABA in mammalian tissues other than the tissues of the nervous system.
Function
In the vertebrates, GABA acts at inhibitory synapses in the brain by binding to specific transmembrane receptors in the plasma membrane of both pre- and postsynaptic neurons. This binding causes the opening of ion channels to allow the flow of either negatively-charged chloride ions into the cell or positively-charged potassium ions out of the cell. This action results in a negative change in the transmembrane potential, usually causing hyperpolarization. Three general classes of GABA receptor are known: GABAAand GABAC ionotropic receptors, which are ion channels themselves, and GABAB metabotropic receptors, which are G protein-coupled receptors that open ion channels via intermediaries (G proteins).
Neurons that produce GABA as their output are called GABAergic neurons, and have chiefly inhibitory action at receptors in the adult vertebrate. Medium Spiny Cells are a typical example of inhibitory CNS GABAergic cells. GABA exhibits excitatory actions in insects, mediating muscle activation at synapses between nerves and muscle cells, and also the stimulation of certain glands. In hippocampus and neocortex of the mammalian brain, GABA has primarily excitatory effects early in development, and is in fact the major excitatory neurotransmitter in many regions of the brain prior to the maturation of glutamate synapses - See developing cortex. Whether GABA is excitatory or inhibitory depends on the direction (into or out of the cell) and magnitude of the ionic currents controlled by the GABAAreceptor. When net positive ionic current is directed into the cell, GABA is excitatory, when the net positive current is directed out of the cell, GABA is inhibitory. A developmental switch in the molecular machinery controlling the polarity of this current is responsible for the changes in the functional role of GABA between the neonatal and adult stages.
In spastic cerebral palsy in humans, GABA cannot be absorbed properly by the damaged nerve rootlets leading to certain muscles; this leads to hypertonia in those muscles.
Structure and conformation
GABA is found mostly as a zwitterion, that is, with the carboxyl group deprotonated and the amino group protonated. Its conformation depends on its environment. In the gas phase, a highly folded conformation is strongly favored due to the electrostatic attraction between the two functional groups. The stabilization is about 50 kcal/mol, according to quantum chemistry calculations. In the solid state, a more extended conformation is found, with a trans conformation at the amino end and a gauche conformation at the carboxyl end. This is due to the packing interactions with the neighboring molecules. In solution, five different conformations, some folded and some extended are found as a result of solvation effects. The conformational flexibility of GABA is important for its biological function, as it has been found to bind to different receptors with different conformations. Many GABA analogues with pharmaceutical applications have more rigid structures in order to control the binding better.[1][2]
History
Gamma-aminobutyric acid was first synthesized in 1883, and was first known only as a plant and microbe metabolic product. In 1950, however, GABA was discovered to be an integral part of the mammalian central nervous system.[3]
Synthesis
Organisms synthesize GABA from glutamate using the enzyme L-glutamic acid decarboxylase and pyridoxal phosphate as a cofactor. It is worth noting that this process converts the principal excitatory neurotransmitter (glutamate) into the principal inhibitory one (GABA).
Pharmacology
Drugs that act as agonists of GABA receptors (known as GABA analogues or GABAnergic drugs) or increase the available amount of GABA typically have relaxing, anti-anxiety and anti-convulsive effects. Many of the substances below are known to cause anterograde amnesia and retrograde amnesia.
GABA has been purported to increase the amount of the Human Growth Hormone. The results of those studies have been seldom replicated, and have recently been in question since it is unknown whether GABA can pass the blood-brain barrier.
References
- Devashis Majumdar and Sephali Guha. Conformation, electrostatic potential and pharmacophoric pattern of GABA (gamma-aminobutyric acid) and several GABA inhibitors. Journal of Molecular Structure: THEOCHEM 1988, 180, 125-140. doi:10.1016/0166-1280(88)80084-8
- Anne-Marie Sapse. Molecular Orbital Calculations for Amino Acids and Peptides. Birkhäuser, 2000. ISBN 0817638938.
- Roth, Robert J.; Cooper, Jack R.; Bloom, Floyd E. (2003). The Biochemical Basis of Neuropharmacology. Oxford [Oxfordshire]: Oxford University Press, 416 pages. ISBN 0-19-514008-7.
- Dzitoyeva S, Dimitrijevic N, Manev H (2003). "Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5485-90. doi:10.1073/pnas.0830111100. PMID 12692303.
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997). "Sites of alcohol and volatile anaesthetic action on GABAA and
- glycine receptors". Nature 389 (6649): 385-9. doi:10.1038/38738.
- Boehm SL, Ponomarev I, Blednov YA, Harris RA (2006). "From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions". Adv. Pharmacol. 54: 171-203. doi:10.1016/j.bcp.2004.07.023.
- Granger P, Biton B, Faure C, Vige X, Depoortere H, Graham D, Langer SZ, Scatton B, Avenet P (1995). "Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin". Mol. Pharmacol. 47 (6): 1189–96. PMID 7603459.
- Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H (2005). "Drosophila GABAB receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". Eur. J. Pharmacol. 519 (3): 246-52. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
- Hunter, A (2006). "Kava (Piper methysticum) back in circulation". Australian Centre for Complementary Medicine25 (7): 529.
Molecules of Life Resources
The Glucose Molecule
Glucose is the most important carbohydrates and is used as a source of energy in animals and plants. Glucose is also one of the main products of photosynthesis and starts respiration..