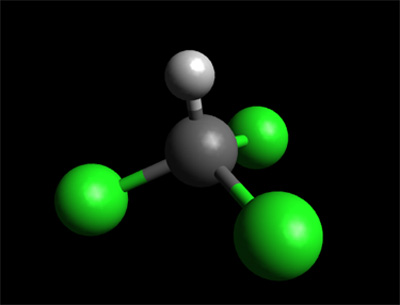
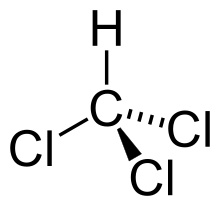
Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid t It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested. (See Development of Ether and Chloroform).
Structure
The molecule adopts tetrahedral molecular geometry similar to that in methane.
History
Chloroform was synthesized independently by several investigators circa 1831: Moldenhawer, a German pharmacist from Frankfurt an der Oder, appears to have produced chloroform in 1830 by mixing chlorinated lime (calcium hypochlorite) with ethanol; however, he mistook it for Chloräther (chloric ether, 1,2-dichloroethane).
Samuel Guthrie, an American physician from Sackets Harbor, New York, also appears to have produced chloroform in 1831 by reacting chlorinated lime with ethanol, as well as noting its anaesthetic properties; however, he also believed that he had prepared chloric ether.
Justus von Liebig carried out the alkaline cleavage of chloral.
Eugène Soubeiran obtained the compound by the action of chlorine bleach on both ethanol and acetone.
In 1834, French chemist Jean-Baptiste Dumas determined chloroform's empirical formula and named it.
In 1835, Dumas prepared the substance by the alkaline cleavage of trichloroacetic acid. Regnault prepared chloroform by chlorination of chloromethane. In 1842, Robert Mortimer Glover in London discovered the anaesthetic qualities of chloroform on laboratory animals.
In 1847, Scottish obstetrician James Y. Simpson was the first to demonstrate the anaesthetic properties of chloroform on humans and helped to popularise the drug for use in medicine. By the 1850s, chloroform was being produced on a commercial basis by using the Liebig procedure, which retained its importance until the 1960s. Today, chloroform — along with dichloromethane — is prepared exclusively and on a massive scale by the chlorination of methane and chloromethane.
Use as a Solvent
The hydrogen attached to carbon in chloroform participates in hydrogen bonding. Worldwide, chloroform is also used in pesticide formulations, as a solvent for fats, oils, rubber, alkaloids, waxes, gutta-percha, and resins, as a cleansing agent, grain fumigant, in fire extinguishers, and in the rubber industry. CDCl3 is a common solvent used in NMR spectroscopy.
Chloroform as an Anesthetic
The anaesthetic qualities of chloroform were first described in 1842 in a thesis by Robert Mortimer Glover, which won the Gold Medal of the Harveian Society for that year. Glover also undertook practical experiments on dogs to prove his theories. Glover further refined his theories and presented them in the thesis for his doctorate at the University of Edinburgh in the summer of 1847. The Scottish obstetrician James Young Simpson was one of the persons required to read the thesis, but later claimed to have never read the thesis and to have come to his conclusions independently. In May 1848, Robert Halliday Gunning made a presentation to the Medico-Chirurgical Society of Edinburgh following a series of laboratory experiments on rabbits that confirmed Glover's findings and also refuted Simpson's claims of originality. The use of chloroform during surgery expanded rapidly thereafter in Europe. In the 1850s, chloroform was used during the birth of Queen Victoria's last two children.