2-arachidonoylglycerol (2-AG) Molecule
Ball and Stick Model for 2-arachidonoylglycerol (2-AG) Molecule
Overview
The endocannabinoid system was revealed from the mechanism of action of marijuana's major psychotropic component , Δ9-tetrahydrocannabinol, and includes two G-protein-coupled receptors (GPCRs; the cannabinoid CB1 and CB2 receptors). Two endogenous ligands for cannabinoid CB1 receptors, anandamide (N-arachidonoylethanolamine) and 2-arachidonoylglycerol (2-AG), have been identified and characterized. 2-AG is the most prevalent endogenous cannabinoid ligand in the brain, and electrophysiological studies suggest 2-AG, rather than anandamide, is the true natural ligand for cannabinoid receptors and the key endocannabinoid involved in retrograde signaling in the brain. (1)
2-AG levels reach in average 12 nmol/g, while the concentration of AEA is 19 pmol/g in rat whole brain (Reference 2). 2-AG is 100- to 1000-fold more abundant than AEA, depending on extraction protocols, tissues, and species.
Unlike anandamide, formation of 2-AG is calcium-dependent and is mediated by the activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) (Reference 3). 2-AG acts as a full agonist at the CB1 receptor (4).
Given these facts, it has been proposed that 2-AG is the 'primary endogenous ligand' for CBRs in the central nervous system (CNS). However, AEA has been shown to activate TRPV1, inhibit l-type Ca2+channels independently, as well as negatively regulate 2-AG biosynthesis and physiological effects in striatum, underscoring its essential role in the regulation of synaptic transmission . (5)
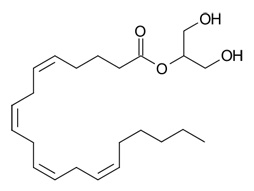
Molecular Structure of 2-arachidonoylglycerol
"...2-AG was discovered by Raphael Mechoulam and his student Shimon Ben-Shabat at Jerusalem University. 2-AG was a known chemical compound but its occurrence in mammals and its affinity for the cannabinoid receptors were first described in 1994-1995. A research group at Teikyo University reported the affinity of 2-AG for the cannabinoid receptors in 1994-1995, but the isolation of 2-AG in the canine gut was first reported in 1995 by the research group of Raphael Mechoulam at the Hebrew University of Jerusalem, which additionally characterized its pharmacological properties in vivo..." (wikipedia and references therein)
Biosynthesis and degradation
2‐Arachidonoylglycerol (2‐AG) is a monoacylglycerol (MAG) molecule containing an esterified arachidonic acid chain at sn‐2 position of the glycerol backbone. Most 2-AG is synthesized from membrane phospholipids via sequential activation of a phospholipase Cβ and a diacylglycerol lipase, although other pathways may contribute in specialized settings. 2-AG breakdown is more complicated with at least eight different enzymes participating. These enzymes can either degrade 2-AG into its components, arachidonic acid and glycerol, or transform 2-AG into highly bioactive signal molecules.(See Reference 6)
Mechanism of Action
The basal level of 2-AG is approximately 1000 times higher than AEA in the brain. Through pharmacological manipulations, altered metabolism of 2-AG, but not AEA, exerts remarkable effects in endocannabinoid-mediated retrograde signaling. Given these facts, it is proposed that 2-AG is the primary endogenous ligand for both CB1 and CB2 in the central nervous system (CNS) (5). Similar to anandamide, 2-AG ia thought to play an important role in the regulation of appetite, immune system functions and pain management.
REFERENCES
- Zuzana Justinová, Sevil Yasar, Godfrey H. Redhi and Steven R. Goldberg. The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys. Journal of Neuroscience 11 May 2011, 31 (19) 7043-7048
- Matthew W Buczynski, Loren Parsons . Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. British Journal of pharmacology (2010).
- Sugiura T, Kodaka T, Nakane S, et al. (January 1999). "Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds". The Journal of Biological Chemistry. 274 (5): 2794–801.
- Savinainen JR, Järvinen T, Laine K, Laitinen JT (October 2001). "Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes". British Journal of Pharmacology. 134 (3): 664–72.
- Shenglong Zou and Ujendra Kumar. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System . Int J Mol Sci. 2018 Mar; 19(3): 833.
- N Murataeva, A Straiker, and K Macki.x Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014 Mar; 171(6): 1379–1391.