Ammonium Hydroxide - Molecule
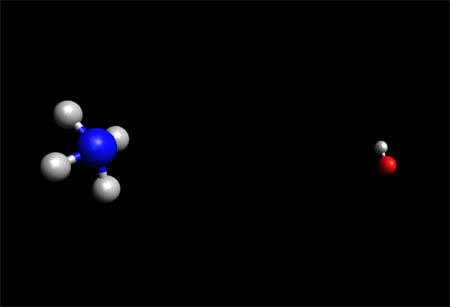
Ammonium Hydroxide Molecule-- Space Fill Model
To View the Ammonia Molecule in 3D using Jsmol
What is pure Ammonia?
At room temperature, ammonia is a colorless, highly irritating gas with a pungent, suffocating odor. In pure form, it is known as anhydrous ammonia and is hygroscopic (readily absorbs moisture).
Ammonia has alkaline properties and is corrosive. Ammonia gas dissolves easily in water to form ammonium hydroxide,...
What is Ammonium Hydroxide?
Ammonium hyroxide also called ammonia water is a solution of ammonia in water. It can be denoted by the symbols NH3(aq)
Mechanism of Production
In aqueous solution, ammonia deprotonates a small fraction of the water to give ammonium and hydroxide according to the following equilibrium:
- NH3 + H2O ⇌ NH4+ + OH−.
Uses of Ammonia Hydroxide
Household cleanerDilute (1–3%) ammonia is also an ingredient of numerous cleaning agents, including many window cleaning formulas. In addition to use as an ingredient in cleansers with other cleansing ingredients, ammonia in water is also sold as a cleaning agent by itself, usually labeled as simply "ammonia". It may be sold plain, lemon-scented (and typically colored yellow), or pine-scented (green). Commonly available ammonia with soap added is known as "cloudy ammonia".
Food production
Baking ammonia (Ammonia Carbonate) was one of the original chemical leavening agents. It was obtained from deer antlers. It is useful as a leavening agent because ammonium carbonate is heat activated. This characteristic allows bakers to avoid both yeast's long proofing time and the quick [CO2] dissipation of baking soda in making breads and cookies rise. It is still used to make ammonia cookies and other crisp baked goods, but its popularity has waned because of ammonia's off putting smell and concerns over its use as a food ingredient compared to modern day baking powder formulations. It has been identified in the E number series as E527. Aqueous ammonia is used as an acidity regulator to bring down the acid levels in food. It is classified in the United States by the Food and Drug Administration as generally recognized as safe (GRAS) when using the food grade version. Its pH control abilities make it an effective antimicrobial agent.
See Also: What is an Acid? What is a Base? What is pH?
Molecules of Life Resources
Common Acids
- Hydrochloric Acid
- Acetic Acid
- Nitric Acid
- Boric Acid
- Carbonic Acid
- Citric Acid
- Sulfuric Acid
- Lactic Acid
Common Bases
- Ammonia (Ammonium hydroxide)
- Potassium Hydroxide
- Calcium Hydroxide
- Sodium bicarbonate
- Magnesium Hydroxide
- Sodium Hydroxide