Citric Acid Molecule
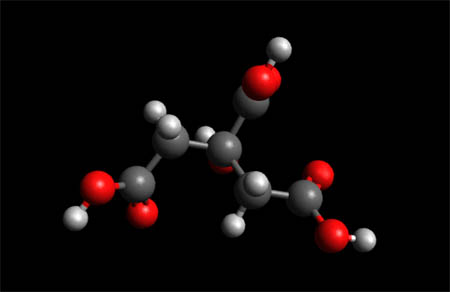
Citric Acid Molecule-- Space Fill Model
To View the Citric Acid Molecule in 3D using Jsmol
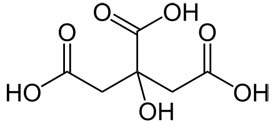
Citric acid is a weak organic acid that has the chemical formula C6H8O7.
Citric acid naturally in citrus fruits. It is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
Presence in Fruits
Citric acid exists in greater than trace amounts in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/l in the juices [1]). The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes. Within species, these values vary depending on the cultivar and the circumstances in which the fruit was grown.
Citric Acid Cycle
The citric acid cycle (CAC) – also known as the TCA cycle (tricarboxylic acid cycle) or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins, into adenosine triphosphate (ATP) and carbon dioxide. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.[2] Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three segments of the citric acid cycle have been recognized.[3]
Commercial Use
More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring and chelating agent.[4].
Powdered citric acid being used to prepare lemon pepper seasoning Because it is one of the stronger edible acids, the dominant use of citric acid is as a flavoring and preservative in food and beverages, especially soft drinks and candies. Within the European Union it is denoted by E number E330. Citric acid has 247 kcal per 100 g.[5] The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals. In the United States the purity requirements for citric acid as a food additive are defined by the Food Chemicals Codex, which is published by the United States Pharmacopoeia (USP). Citric acid can be added to ice cream as an emulsifying agent to keep fats from separating, to caramel to prevent sucrose crystallization, or in recipes in place of fresh lemon juice. Citric acid is used with sodium bicarbonate in a wide range of effervescent formulae, both for ingestion (e.g., powders and tablets) and for personal care (e.g., bath salts, bath bombs, and cleaning of grease). Citric acid sold in a dry powdered form is commonly sold in markets and groceries as "sour salt", due to its physical resemblance to table salt. It has use in culinary applications, as an alternative to vinegar or lemon juice, where a pure acid is needed. Citric acid can be used in food coloring to balance the pH level of a normally basic dye.
See Also: What is an Acid? What is a Base? What is pH?
References
1- Penniston KL, Nakada SY, Holmes RP, Assimos DG; Nakada; Holmes; Assimos (2008). "Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products". Journal of Endourology. 22 (3): 567–570. doi:10.1089/end.2007.0304. PMC 2637791. PMID 18290732.
2- Lane, Nick (2009). Life Ascending: The Ten Great Inventions of Evolution. New York: W. W. Norton & Co. ISBN 978-0-393-06596-1.
3- Chinopoulos, Christos (August 2013). "Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex". Journal of Neuroscience Research. 91 (8): 1030–43. doi:10.1002/jnr.23196. PMID 23378250.
4- Apleblat, Alexander (2014). Citric acid. Springer. ISBN 978-3-319-11232-9.
5- Greenfield, Heather; Southgate, D.A.T. (2003). Food Composition Data: Production, Management and Use. Rome: FAO. p. 146.
Molecules of Life Resources
Common Acids
- Hydrochloric Acid
- Acetic Acid
- Nitric Acid
- Boric Acid
- Carbonic Acid
- Citric Acid
- Sulfuric Acid
- Lactic Acid
Common Bases
- Ammonia (Ammonium hydroxide)
- Potassium Hydroxide
- Calcium Hydroxide
- Sodium bicarbonate
- Magnesium Hydroxide
- Sodium Hydroxide