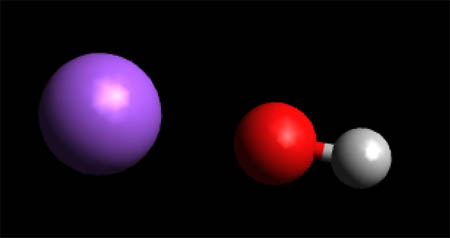
Sodium Hydroxide - Molecule
Sodium Hydroxide Molecule-- Space Fill Model
To View the Sodium Hyroxide in 3D using Jsmol
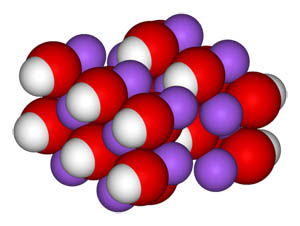
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH− . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH 2O.[1] The monohydrate NaOH·H2O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, it is frequently utilized alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.[2]
Sodium Hydroxide is highly soluble in water, with a lower solubility in polar solvents such as ethanol and methanol.[3]
See Also: What is an Acid? What is a Base? What is pH?
References
1- P. R. Siemens, William F. Giauque (1969): "Entropies of the hydrates of sodium hydroxide. II. Low-temperature heat capacities and heats of fusion of NaOH·2H2O and NaOH·3.5H2O". Journal of Physical Chemistry, volume 73, issue 1, pages 149–157. doi:10.1021/j100721a024
2- "Examples of Common Laboratory Chemicals and their Hazard Class".
"3- Sodium Hydroxide – Compound Summary". Retrieved Feb. 13, 2019.
Molecules of Life Resources
Common Acids
- Hydrochloric Acid
- Acetic Acid
- Nitric Acid
- Boric Acid
- Carbonic Acid
- Citric Acid
- Sulfuric Acid
- Lactic Acid