Sodium Bicarbonate - Molecule
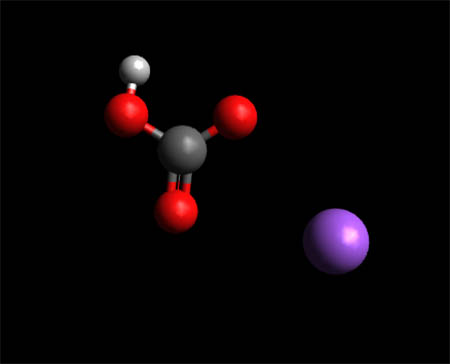
Sodium Bicarbonate Molecule-- Space Fill Model
To View the Sodium Bicarbonate in 3D using Jsmol
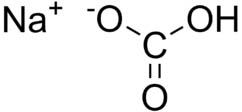
Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). It has a slightly salty, alkaline taste. Sodium bicarbonate is produced industrially from sodium carbonate also known as soda ash.
Na2CO3 + CO2 + H2O → 2 NaHCO3Medical uses and health
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.[26] Its reaction with stomach acid (HCl) produces salt, water, and carbon dioxide:
- NaHCO3 + HCl → NaCl + H2O + CO2(g)
A mixture of sodium carbonate and polyethylene glycol such as PegLyte[1], dissolved in water and taken orally, is an effective gastrointestinal lavage preparation and laxative prior to gastrointestinal surgery, colenoscopy etc.
Intravenous sodium bicarbonate in an aqueous solution is sometimes used for cases of acidosis, or when insufficient sodium or bicarbonate ions are in the blood. [2] In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left, and thus raises the pH. For this reason, sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is markedly low (< 7.1–7.0). [3] It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).[4]
As early as the 1920s, bicarbonate was found to cause increased bone strength in patients who were losing calcium in their urine. In 1968, diets producing too much acid were thought to put bones at risk. Experiments by Anthony Sebastian of the University of California, San Francisco starting in the late 20th century found that the body was breaking down bones and muscles to release carbonates, phosphates, and ammonia, which neutralize acid. Adding bicarbonate to the diet (Note: he used potassium bicarbonate) reduced loss of calcium in postmenopausal women, amounting to the equivalent of "an arm-and-a-leg's worth" of bone if this continued for two decades. See:
Adding bicarbonate to your diet through fruits and vegetables leads to greater bone density, according to a 2009 article.In a recent study published in the Journal of Immunology, oral baking soda was found to activate a splenic anti-inflammatory pathway that seems to reduce the destructive inflammation of autoimmune diseases such as rheumatoid arthritis. Proinflammatory molecules were reduced and anti-inflammatory molecules were released. [5]. See also in Nutrition review: Baking Soda Reduces Inflammation of Rheumatoid Arthritis, Other Autoimmune Disease.
Medical Warnings on Sodium Bicarbonate
Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions; in patients with renal insufficiency, especially those with severe insufficiency such as oliguria or anuria; and in patients receiving corticosteroids or corticotropin, since each gram of sodium bicarbonate contains about 12 mEq of sodium. IV administration of sodium bicarbonate may cause fluid and/or solute overload resulting in dilution of serum electrolytes, overhydration, congestive conditions, or pulmonary edema. see reference Drug Warning 7.6.
Personal hygiene
Toothpaste containing sodium bicarbonate has in several studies been shown to have a better whitening[45][45][46][47] and plaque removal effect[48][49] than toothpastes without it.
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties. It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections. 6-7-8 Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant. Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.[55]
It is used in eye hygiene to treat blepharitis. This is done by addition of a teaspoon of sodium bicarbonate to cool water that was recently boiled, followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.[56]
See Also: What is an Acid? What is a Base? What is pH?
References
1- PegLyte
2."Sodium Bicarbonate Intravenous Infusion" (PDF). Consumer Medicine Information. Better Health Channel. 2004-07-13. See: original (PDF) on 2008-08-22.
3. "Respiratory Acidosis: Treatment & Medication". emedicine.
4-"Insect bites and stings: First aid". Mayo Clinic. 2008-01-15.
6. Storehagen, Silje; Ose, Nanna and Midha, Shilpi. "Dentifrices and mouthwashes ingredients and their use" (PDF). Institutt for klinisk odontologi. Universitetet i Oslo.
7. Barth, Jordan. Oral Product. US Patent US4132770A, filed 1977, and issued 1979.
8.K. Iqbal et al., "Role of Different Ingredients of Tooth Pastes and Mouthwashes in Oral Health.," JPDA (Journal of Pakistan Dental Association) 20, no. 03 (Summer 2011): 164, accessed September 29, 2018.
Molecules of Life Resources
Common Acids
- Hydrochloric Acid
- Acetic Acid
- Nitric Acid
- Boric Acid
- Carbonic Acid
- Citric Acid
- Sulfuric Acid
- Lactic Acid