MitoQ Molecule
Image: MitoQ - Ball and Stick Model
To View the MitoQ Molecule in 3D --->> with Jsmol
Beta MitoQ is an antioxidant that targets the mitochondria in living cells in order to protect them from oxidative damage. Mitochondria are an essential oranelle in most cells that use oxygen to break down molecular carbohydrates and fat to produce energy in the form of ATP. MitoQ has undergone clinical trials in humans. Most recently it has been shown that ".." Older adults who take a novel antioxidant molecule MitoQ that specifically targets cellular powerhouses, or mitochondria, see age-related vascular changes reverse by the equivalent of 15 to 20 years within six weeks, according to new University of Colorado Boulder research. .." see article.
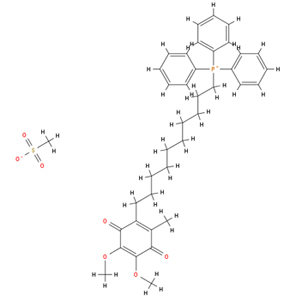
MitoQ Molecular Structure
Reactive Oxygen Species (ROS) formed in Mitochondria
In a biological context, ROS are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling and homeostasis. Examples include peroxides, superoxide, hydroxyl radical, and singlet oxygen. However, during times of environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically. This may result in significant damage to cell structures. Cumulatively, this is known as oxidative stress.
ROS are produced intracellularly through multiple mechanisms. Mitochondria convert energy for the cell into a usable form, adenosine triphosphate (ATP). The process in which ATP is produced, called oxidative phosphorylation, involves the transport of protons (hydrogen ions) across the inner mitochondrial membrane by means of the electron transport chain. In the electron transport chain, electrons are passed through a series of proteins via oxidation-reduction reactions, with each acceptor protein along the chain having a greater reduction potential than the previous. The last destination for an electron along this chain is an oxygen molecule. In normal conditions, the oxygen is reduced to produce water; however, in about 0.1–2% of electrons passing through the chain, oxygen is instead prematurely and incompletely reduced to give the superoxide radical (•O− 2).While superoxide radical itself is only moderately reactive it can easily be converted into more damaging molecules.
Mitochondria are the major source of the free radical superoxide within cells, mitochondrial oxidative stress is thought to be a major contributing factor underlying a wide range of diseases and pathologies including: heart attack, stroke and sepsis, and also chronic disorders such as diabetes, metabolic syndrome, inflammation, and many of the degenerative processes and diseases associated with ageing such as Parkinson's disease and Alzheimer's disease.
Design and Sythesis of MitoQ
Many antioxidants, which are designed to block the damage caused by reactive oxygen species, should be effective therapies for a wide range of diseases by decreasing mitochondrial oxidative damage
MitoQ was designed in the late 1990s as a mitochondria-targeted antioxidant by Michael P. Murphy and Robin A. J. Smith, and synthesised by Geoffrey Kelso., at the University of Otago, Dunedin, New Zealand.
MitoQ was designed to accumulate extensively within mitochondria in vivo in order to increase the local antioxidant capacity and thereby decrease mitochondrial oxidative damage. To do this MitoQ incorporates a lipophilic cation, that is a positively charged component that is sufficiently hydrophobic, or “oily”, to be able to pass directly through biological membranes. The lipophilic cation used is based on the triphenylphosphonium structure, which is well known to accumulate within the negative mitochondrial matrix. In the solid form of MitoQ the positive charge is neutralised with a negatively charged anion, typically mesylate, to form a salt. MitoQ is present in two different forms, the oxidised ubiquinone form, Mitoquinone and the reduced ubiquinol form, Mitoquinol. MitoQ can refer to either form or to a mixture of the forms.
Uptake of Lipophilic MitoQ by Mitochondria
The uptake of lipophilic cations into mitochondria occurs because of the large membrane potential or voltage across the mitochondrial inner membrane. Lipophilic cations have a number of unusual properties in that while they are water-soluble they can still easily pass through the hydrophobic core of biological membranes and therefore do not require specific protein carriers.
The uptake of lipophilic cations such as MitoQ into mitochondria within cells will be driven by the membrane potential across the plasma membrane (30–60 mV) and by the mitochondrial membrane potential (150–180 mV); consequently, the MitoQ concentration within mitochondria will be about a thousandfold greater than that outside the cell. This was confirmed by studies which measured the uptake of MitoQ by both isolated mitochondria and by mitochondria within cells.
Antioxidant Properties of MitoQ
The antioxidant component of MitoQ is the same ubiquinone as found in Coenzyme Q 10 . Within mitochondria the ubiquinone part of MitoQ is rapidly activated to the active ubiquinol antioxidant by the action of the enzyme Complex II (also called succinate dehydrogenase) in the mitochondrial respiratory chain. After detoxifying a free radical or other ROS the ubiquinol part of MitoQ is converted back to ubiquinone, which is then recycled back to the active antioxidant by Complex II within the mitochondria. It is this combination of a thousand-fold concentration within mitochondria, coupled to its conversion to the active antioxidant and recycling back to the active antioxidant after detoxification of a free radical that makes MitoQ such an effective mitochondria-targeted antioxidant.
Molecules of Life Resources
Antioxidant Molecules
- Astaxanthin
- Ellagic Acid
- Picrocrocin
- Lycopene
- Lutein/Zeaxanthin
- Quercetin
- Vitamin C
- IP-6
- Beta-Carotene
- Epicatechin
- ECGC- Epigallocatechin gallate
- Safranal
- Proanthocyanidins
- Resveratrol
- Vitamin E
- Melatonin
- MitoQ