Insulin Molecule
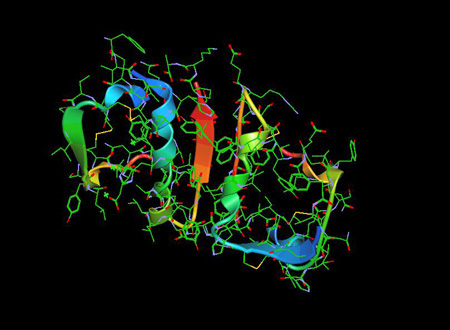
The Insulin Molecule 1ZNI PDB is a pancreatic hormone that plays an essential role in regulation of blood glucose as well as lipid and carbohydrate metabolism..
For 3-D Structure of the Insulin Molecule using Jsmol Click
Overview
Insulin is a polypeptide hormone produced by pancreatic islet β cells that is primarily responsible for regulation of blood glucose and storage of carbohydrates and lipids. Type 1 diabetes is due to inadequate production of insulin caused by destruction and loss of insulin producing pancreatic islet β cells. Type 2 diabetes is due to relative insulin resistance. It is considered to be the main anabolic hormone of the body. (1)
Protein Structure
Insulin was found to be a polypeptide in 1928 with its amino acid sequence identified in 1952. It is in fact a dipeptide, containing A and B chains respectively, linked by disulphide bridges, and containing 51 amino acids, with a molecular weight of 5802. Its iso-electric point is pH 5.5.5 The A chain comprises 21 amino acids and the B chain 30 amino acids. The A chain has an N-terminal helix linked to an anti-parallel C-terminal helix; the B chain has a central helical segment. The two chains are joined by 2 disulphide bonds, which join the N- and C-terminal helices of the A chain to the central helix of the B chain. In pro-insulin, a connecting peptide links the N-terminus of the A chain to the C-terminus of the B chain. [2]
Within vertebrates, the amino acid sequence of insulin is strongly conserved. Bovine insulin differs from human in only three amino acid residues, and porcine insulin in one. Even insulin from some species of fish is similar enough to human to be clinically effective in humans. Insulin in some invertebrates is quite similar in sequence to human insulin, and has similar physiological effects. The strong homology seen in the insulin sequence of diverse species suggests that it has been conserved across much of animal evolutionary history. The C-peptide of proinsulin, however, differs much more among species; it is also a hormone, but a secondary one.
Insulin is produced and stored in the body as a hexamer (a unit of six insulin molecules), while the active form is the monomer. The hexamer is an inactive form with long-term stability, which serves as a way to keep the highly reactive insulin protected, yet readily available. The hexamer-monomer conversion is one of the central aspects of insulin formulations for injection. The hexamer is far more stable than the monomer, which is desirable for practical reasons; however, the monomer is a much faster-reacting drug because diffusion rate is inversely related to particle size.
Mechanisms of Insulin Secretion
Insulin is produced in the pancreas and the Brockmann body (in some fish), and released when any of several stimuli are detected. These stimuli include ingested protein and glucose in the blood produced from digested food.[3] Carbohydrates can be polymers of simple sugars or the simple sugars themselves. If the carbohydrates include glucose, then that glucose will be absorbed into the bloodstream and blood glucose level will begin to rise. In target cells, insulin initiates a signal transduction, which has the effect of increasing glucose uptake and storage. Finally, insulin is degraded, terminating the response.
In mammals, insulin is synthesized in the pancreas within the beta cells. One million to three million pancreatic islets form the endocrine part of the pancreas, which is primarily an exocrine gland. The endocrine portion accounts for only 2% of the total mass of the pancreas. Within the pancreatic islets, beta cells constitute 65–80% of all the cells.
Release of Insulin
Blood glucose regulation Beta cells in the islets of Langerhans release insulin in two phases. The first-phase release is rapidly triggered in response to increased blood glucose levels, and lasts about 10 minutes. The second phase is a sustained, slow release of newly formed vesicles triggered independently of sugar, peaking in 2 to 3 hours. Reduced first-phase insulin release may be the earliest detectable beta cell defect predicting onset of type 2 diabetes.[4] First-phase release and insulin sensitivity are independent predictors of diabetes.[5]
Blood Insulin Levels
The blood insulin level can be measured in international units, such as µIU/mL or in molar concentration, such as pmol/L, where 1 µIU/mL equals 6.945 pmol/L.[6] A typical blood level between meals is 8–11 μIU/mL (57–79 pmol/L).[7]
Mechanism of Action -Signal Transduction of Insulin
Signal transduction[edit] The effects of insulin are initiated by its binding to a receptor present in the cell membrane. The receptor molecule contains an α- and β subunits. Two molecules are joined to form what is known as a homodimer. Insulin binds to the α-subunits of the homodimer, which faces the extracellular side of the cells. The β subunits have tyrosine kinase enzyme activity which is triggered by the insulin binding. This activity provokes the autophosphorylation of the β subunits and subsequently the phosphorylation of proteins inside the cell known as insulin receptor substrates (IRS). The phosphorylation of the IRS activates a signal transduction cascade that leads to the activation of other kinases as well as transcription factors that mediate the intracellular effects of insulin.[8]
Action of Insulin of Physiological Effects
he actions of insulin on the global human metabolism level include: Increase of cellular intake of certain substances, most prominently glucose in muscle and adipose tissue (about two-thirds of body cells) [9] Increase of DNA replication and protein synthesis via control of amino acid uptake Modification of the activity of numerous enzymes.
Regulator of endocannabinoid metabolism. Insulin is a major regulator of endocannabinoid (EC) metabolism and insulin treatment has been shown to reduce intracellular ECs, the 2-arachidonylglycerol (2-AG) and anandamide (AEA), which correspond with insulin-sensitive expression changes in enzymes of EC metabolism. In insulin-resistant adipocytes, patterns of insulin-induced enzyme expression is disturbed in a manner consistent with elevated EC synthesis and reduced EC degradation. Findings suggest that insulin-resistant adipocytes fail to regulate EC metabolism and decrease intracellular EC levels in response to insulin stimulation, whereby obese insulin-resistant individuals exhibit increased concentrations of ECs.[10] This dysregulation contributes to excessive visceral fat accumulation and reduced adiponectin release from abdominal adipose tissue, and further to the onset of several cardiometabolic risk factors that are associated with obesity and type 2 diabetes.[11]
References
1- Voet D, Voet JG (2011). Biochemistry (4th ed.). New York: Wiley.
2-Gisela Wilcox (2005) Insulin and Insulin Resistance, Clin Biochem Rev. 2005 May; 26(2): 19–39.
3- Rhoades RA, Bell DR (2009). Medical physiology : principles for clinical medicine (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 644–47.
4-Gerich JE (February 2002). "Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes?". Diabetes. 51 (Suppl 1): S117–21. doi:10.2337/diabetes.51.2007.s117. PMID 11815469.
5- Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM (September 2010). "Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS)". Diabetes
6- A Dictionary of Units of Measurement Archived 2013-10-28 at the Wayback Machine. By Russ Rowlett, the University of North Carolina at Chapel Hill. June 13, 2001
7- Iwase H, Kobayashi M, Nakajima M, Takatori T (January 2001). "The ratio of insulin to C-peptide can be used to make a forensic diagnosis of exogenous insulin overdosage". Forensic Science International. 115 (1–2): 123–27. doi:10.1016/S0379-0738(00)00298-X. PMID 11056282.
8- Xiang Z (June 2006). "Advances in homology protein structure modeling". Current Protein & Peptide Science. 7 (3): 217–27. doi:10.2174/138920306777452312. PMC 1839925. PMID 16787261.
9- Zagrovic B, Snow CD, Shirts MR, Pande VS (November 2002). "Simulation of folding of a small alpha-helical protein in atomistic detail using worldwide-distributed computing". Journal of Molecular Biology. 323 (5): 927–37.
10-D'Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR (May 2008). "The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids". Diabetes. 57 (5): 1262–68.
11- Di Marzo V (August 2008). "The endocannabinoid system in obesity and type 2 diabetes". Diabetologia. 51 (8): 1356–67. doi:10.1007/s00125-008-1048-2. PMID 18563385.
Molecules of Life Resources
The Glucose Molecule
Glucose is the most important carbohydrates and is used as a source of energy in animals and plants. Glucose is also one of the main products of photosynthesis and starts respiration..