Resveratrol as a Senolytic and Anti-Aging Molecule
Ball and Stick Model for Resveratrol Molecule
To View the Resveratrol Molecule in 3D --->> with Jsmol
Molecular formula C14H12O3
Molar mass 228.247 g·mol−1
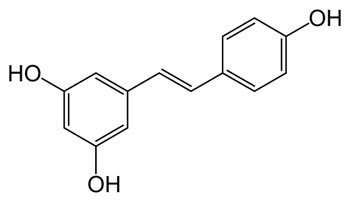
Resveratrol molecular structure
About Resveratrol
Resveratrol is one of a group of compounds (called phytoalexins) that are produced in plants during times of environmental stress such as adverse weather or insect, animal or pathogenic attack. Resveratrol has been identified in more than 70 species of plants, including mulberries and peanuts. Grapes, however are particularly good sources. Resveratrol is found in the skin (not flesh) of grapes. Fresh grape skin contains about 50 to 100 micrograms of resveratrol per gram, while red wine concentrations range from 1.5 to 3 milligrams per liter. This compound is also thought to be responsible, in part, for the cholesterol-lowering effects of red wine and may also explain why those consuming a Mediterranean-type diet (of which red wine consumption is characteristic) may have a reduced risk of heart disease.
In a study by Pezzuto and colleagues (Jang, M., Cai, L., Udeani, G.O., Slowing, K.V., Thomas, C.F., Beecher, C.W.W., Fong, H.H.S., Farnsworth, N.R., Kinghorn, A.D., Mehta, R.G., Moon, R.C. and Pezzuto, J.M. Science volume 10:218-221, 1997) it was demonstrated that resveratrol was effective during all three phases of the cancer process: initiation, promotion and progression. Resveratrol was found to have antioxidant and antimutagenic activity and also increased levels of the phase II drug-metabolizing enzyme quinone reductase, an enzyme capable of metabolically detoxifying carcinogens, thereby ridding them from the body.
Recent results show resveratrol also extends lifespan in yeast --David Sinclair of Harvard Medical School in Boston, Massachusetts, led the research The compound is one of 17 plant molecules so far found to extend life in baker's yeast (Saccharomyces cerevisiae). Resveratrol also gives fruitflies, which typically live for around a month, an extra ten days of life. Sinclair gave his yeast fresh grape extract. Normally, the organism divides around 25 times and then dies. Resveratrol-treated yeast underwent an extra 15 replications.
Resveratrol is a phytoalexin produced naturally by several plants when under attack by bacteria or fungi. Phytoalexins are antibacterial and anti-fungal chemicals produced by plants as a defense against infection by pathogens. Resveratrol has also been produced by chemical synthesis,[1] and is sold as a nutritional supplement derived primarily from Japanese knotweed. A number of beneficial health effects, such as anti-cancer, antiviral, neuroprotective, anti-aging, and anti-inflammatory effects have been reported, but all of these studies are "in-vitro" (test tube) or in yeast, worms, fruit flies, fish, mice, and rats. Resveratrol is found in the skin of red grapes and is a constituent of red wine but, based on extrapolation from animal trials, apparently not in sufficient amounts to explain the "French paradox" that the incidence of coronary heart disease is relatively low in southern France despite high dietary intake of saturated fats.[2]
The four stilbenes cis- and trans-resveratrol, and their glucosides cis- and trans-piceid are sometimes analyzed together as a group.[3]
Chemical and physical properties
Resveratrol (3,5,4'-trihydroxystilbene) is a polyphenolic phytoalexin. It is a stilbenoid, a derivate of stilbene, and is produced in plants with the help of the enzyme stilbene synthase.
It exists as two geometric isomers: cis- (Z) and trans- (E), with the trans-isomer shown in the top image. The trans- form can undergo isomerisation to the cis- form when exposed to ultraviolet irradiation.[4] Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 degrees C in the presence of air.[5] Resveratrol content also stayed stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[6]
Plants and foods isolation
Resveratrol was originally isolated by Takaoka from the roots of white hellebore in 1940, and later, in 1963, from the roots of japanese knotweed. However, it attracted the wider attention only in 1992, when its presence in wine was used as the explanation for cardioprotective effects of wine.[7]
In grapes, resveratrol is found primarily in the skin,[8] and in muscadine grapes also in the seeds.[9] The amount found in grape skins also varies with the grape cultivar, its geographic origin, and exposure to fungal infection. The amount of fermentation time a wine spends in contact with grape skins is an important determinant of its resveratrol content.[8]
The amount of resveratrol in food varies greatly. Red wine contains between 0.2 and 5.8 mg/L,[10] depending on the grape variety, while white wine has much less the reason being that red wine is fermented with the skins, allowing the wine to absorb the resveratrol, whereas white wine is fermented after the skin has been removed.[8] Wines produced from muscadine grapes, however, both red and white, may contain more than 40 mg/L.[11][3]Content in wines and grape juice
| Beverage | Total resveratrol (mg/L)[8][3] | Total resveratrol in a 5 ounce glass (mg)[8][3] |
|---|---|---|
| Muscadine Wines | 14.1 - 40 | 2.12 - 6 |
| Red Wines (Global) | 1.98 - 7.13 | 0.30 - 1.07 |
| Red Wines (Spanish) | 1.92 - 12.59 | 0.29 - 1.89 |
| Red grape juice (Spanish) | 1.14 - 8.69 | 0.17 - 1.30 |
| Rose Wines (Spanish) | 0.43 - 3.52 | 0.06 - 0.53 |
| Pinot Noir | 0.40 - 2.0 | 0.06 - 0.30 |
| White Wines (Spanish) | 0.05 - 1.80 | 0.01 - 0.27 |
The trans-resveratrol concentration in 40 Tuscan wines ranged from 0.3 to 2.1 mg/L in the 32 red wines and had a maximum of 0.1 mg/L in the 8 white wines tested. Both the cis- and trans-isomers of resveratrol were detected in all tested samples. cis-Resveratrol levels were comparable to those of the trans-isomer. They ranged from 0.5 mg/L to 1.9 mg/L in red wines and had a maximum of 0.2 mg/L in white wines.[12]
Reports suggest that some aspect of the wine making process converts piceid to resveratrol in wine, as wine seems to have twice the average resveratrol concentration of the equivalent commercial juices.[3]
"All of the muscadine table wines sampled had greater trans and cis resveratrol concentrations than any other wines sampled. The muscadine table wines varied between 9.2 and 31.9 mg/L cis-resveratrol and between 4.9 and 13.4 mg/L trans-resveratrol."[3]
Content in selected foods
| Food | Serving | Total resveratrol (mg)[13] |
|---|---|---|
| Peanuts (raw) | 1 c (146 g) | 0.01 - 0.26 |
| Peanuts (boiled) | 1 c (180 g) | 0.32 - 1.28 |
| Peanut butter | 1 c (258 g) | 0.04 - 0.13 |
| Red grapes | 1 c (160 g) | 0.24 - 1.25 |
Ounce for ounce, peanuts have more than half the amount of resveratrol in red wine. The average amount of resveratrol in one ounce of peanuts in the marketplace (about 15 whole) is 79.4 µg/ounce.
In comparison, some red wines contain approximately 160 µg/fluid ounce.[14] Resveratrol was detected in grape, cranberry, and wine samples. Concentrations ranged from 1.56 to 1042 nmol/g in Concord grape products, and from 8.63 to 24.84 micromol/L in Italian red wine. The concentrations of resveratrol were similar in cranberry and grape juice at 1.07 and 1.56 nmol/g, respectively.[15]
Blueberries have about twice as much resveratrol as bilberries, but there is great regional variation. These fruits have less than ten percent of the resveratrol of grapes. Cooking or heat processing of these berries will contribute to the degradation of resveratrol, reducing it by up to half. [16]
Supplementation
Resveratrol nutritional supplements, first sourced from ground dried grape skins and seeds (sometimes from residual byproducts of winemaking), are now primarily derived from the cheaper, more concentrated Japanese knotweed which contains up to 187 mg/kg in the dried root.[citation needed]
As a result of extensive news coverage,[17][18] sales of supplements greatly increased in 2006,[19][20] despite cautions that benefits to humans are unproven.[21][20]
Physiological effects
Life extension
The groups of Howitz and Sinclair reported in 2003 in the journal Nature that resveratrol significantly extends the lifespan of the yeast Saccharomyces cerevisiae.[22] Later studies conducted by Sinclair showed that resveratrol also prolongs the lifespan of the worm Caenorhabditis elegans and the fruit fly Drosophila melanogaster.[23] In 2007 a different group of researchers was able to reproduce the Sinclair's results with C. elegans[24] but a third group could not achieve consistent increases in lifespan of Drosophila or C. elegans.[25]
In 2006, Italian scientists obtained the first positive result of resveratrol supplementation in a vertebrate. Using a short-lived fish, Nothobranchius furzeri, with a median life span of nine weeks, they found that a maximal dose of resveratrol increased the median lifespan by 56%. Compared with the control fish at nine weeks, that is by the end of the latter life, the fish supplemented with resveratrol showed significantly higher general swimming activity and better learning to avoid an unpleasant stimulus. The authors noted a slight increase of mortality in young fish caused by resveratrol and hypothesized that it is its weak toxic action that stimulated the defense mechanisms and resulted in the life span extension.[26] Later the same year, Sinclair reported that resveratrol counteracted the detrimental effects of a high-fat diet in mice. The high fat diet was compounded by adding hydrogenated coconut oil to the standard diet; it provided 60% of energy from fat, and the mice on it consumed about 30% more calories then the mice on standard diet. Both the mice fed the standard diet and the high-fat diet plus 22 mg/kg resveratrol had a 30% lower risk of death than the mice on the high-fat diet. Gene expression analysis indicated the addition of resveratrol opposed the alteration of 144 out of 155 gene pathways changed by the high-fat diet. Insulin and glucose levels in mice on the high-fat+resveratrol diet were closer to the mice on standard diet then to the mice on the high-fat diet. However, addition of resveratrol to the high-fat diet did not change the levels of free fatty acids and cholesterol, which were much higher than in the mice on standard diet. [27]
Cancer prevention
In 1997 Jang reported that topical resveratrol applications prevented the skin cancer development in mice treated with a carcinogen.[28]There have since been dozens of studies of the anti-cancer activity of resveratrol in animal models.[7][29] The effectiveness of resveratrol in animal cancer models is limited by its poor bioavailability. The strongest evidence of anti-cancer action of resveratrol exists for the tumors it can come into direct contact with, such as skin and gastrointestinal tract tumors. For other cancers, the evidence is equivocal, even if massive dose of resveratrol are used.[29]
Thus, topical application of resveratrol in mice, both before and after the UVB exposure, inhibited the skin damage and decreased skin cancer incidence. However, oral resveratrol was ineffective in treating mice inoculated with melanoma cells.[29] Resveratrol (1 mg/kg orally) reduced the number and size of the esophageal tumors in rats treated with a carcinogen.[30] In several studies, small doses (0.02-8 mg/kg) of resveratrol, given prophylactically, reduced or prevented the development of intesinal and colon tumors in rats given different carcinogens.[29]
Resveratrol is under extensive investigation as a cancer chemopreventive agent.[31][32] Studies show that small doses of dietary resveratrol can reduce colon carcinogenesis in rats and mice.[33] One German study has already been shown to that under special conditions, resveratrol induces apoptosis in human fat cells. In addition, it inhibits production of cytokines which are involved in the development of obesity-related disorders.[34]
Athletic performance with Resveratrol
Johan Auwerx (at the Institute of Genetics and Molecular and Cell Biology in Illkirch, France) and coauthors published an online article in the journal CELL in November 2006. Mice fed resveratrol for 15 weeks had better treadmill endurance than controls. The study supported Sinclair's hypothesis that the effects of resveratrol are indeed due to the activation of SIRT1.
Nicholas Wade's interview-article with Dr. Auwerx[35] states that the dose was 400 mg/kg of body weight (much higher than the 22 mg/kg of the Sinclair study). For an 80 kg (176 lb) person, the 400 mg/kg of body weight amount used in Dr. Auwerx's mouse study would come to 32,000 mg/day. Compensating for the fact that humans have slower metabolic rates than mice would change the equivalent human dose to roughly 4571 mg/day. Again, there is no published evidence anywhere in the scientific literature of any clinical trial for efficacy in humans. There is limited human safety data (see above). It is premature to take resveratrol and expect any particular results. Long-term safety has not been evaluated in humans.
In a study of 123 Finnish adults, those born with certain increased variations of the SIRT1 gene had faster metabolisms, helping them to burn energy more efficiently indicating that the same pathway shown in the lab mice works in humans too.[36]
Antiviral effects
Resveratrol seems to increase the potency of some antiretroviral drugs against HIV in vitro.[37]
Infection by herpes simplex virus ordinarily activates the cell protein Nuclear Factor κB (NF-κB). A Northeastern Ohio Universities College of Medicine study undertaken in Vero cells found that resveratrol suppresses the activation of this transcription- and apoptosis-related protein. The study further found that multiple viral protein products were reduced or completely blocked, as well as a reduction in viral DNA production.[38]
A cell culture study found that resveratrol blocks the influenza virus from transporting viral proteins to the viral assembly site, hence restricting its ability to replicate. The effect was 90% when resveratrol was added six hours after infection and continued for 24 hours thereafter.[39]
Antinflammatory Effects
"...In what researchers state is the first pilot clinical trial to assess the effects of resveratrol on pain severity and levels of inflammatory biomarkers in patients with mild to moderate knee osteoarthritis, the scientists compared treatment with a nonsteroidal anti-inflammatory drug (NSAID) combined with either resveratrol or placebo over 90 days. Pain severity decreased significantly with resveratrol and blood levels of several inflammatory biomarkers were significantly reduced, accorded to the results published in Journal of Medicinal Food... the orally administered resveratrol, given as an adjuvant with meloxicam, led to a significant reduction in the total pain score and to significantly lower levels of serum biomarkers of inflammation common in knee osteoarthritis including TNF-α, interleukin IL-1β, and IL-6..."see source
Side effects
While the health benefits of resveratrol seem promising, one study has theorized that it may stimulate the growth of human breast cancer cells, possibly because of resveratrol's chemical structure, which is similar to a phytoestrogen.[40][41] However, other studies have found that resveratrol actually fights breast cancer.[42][43] Citing the evidence that resveratrol is estrogenic, some retailers of resveratrol advise that the compound may interfere with oral contraceptives and that women who are pregnant or intending to become pregnant should not use the product, while others advise that resveratrol should not be taken by children or young adults under 18, as no studies have shown how it affects their natural development.[44] A small study found that a single dose of up to 5 g of trans-resveratrol caused no serious adverse effects in healthy volunteers.[45]
There is some evidence that resveratrol might exacerbate West Nile virus, since West Nile is p53 mediated and worsened by increased rates of apoptosis of infected cells. [7]
Mechanisms of action for Resveratrol
The mechanisms of resveratrol's apparent effects on life extension are not fully understood, but they appear to mimic several of the biochemical effects of calorie restriction. A new report indicates that resveratrol activates SIRT1 and PGC-1α and improve functioning of the mitochondria.[46] Other research calls into question the theory connecting resveratrol, SIRT1, and calorie restriction.[47][48]
An article in press as of January 2008 discusses resveratrol action in cells. It reports a 14-fold increase in the action of MnSOD.[49] MnSOD reduces superoxide to H2O2, but H2O2 is not increased due to other cellular activity. Superoxide O2- is a byproduct of respiration in complex 1 and 3 of the electron transport chain. It is "not highly toxic, [but] can extract an electron from biological membrane and other cell components, causing free radical chain reactions. Therefore is it essential for the cell to keep superoxide anions in check."[50] MnSOD reduces superoxide and thereby confers resistance to mitochondrial dysfunction, permeability transition, and apoptotic death in various diseases.[51] It has been implicated in lifespan extension, inhibits cancer (e.g. pancreatic cancer [52][53]), and provides resistance to reperfusion injury and irradiation damage [54] [55] [56]. These effects have also been observed with resveratrol. Ellen et al propose MnSOD is increased by the pathway RESV --> SIRT1 / NAD+ --> FOXO3a --> MnSOD. Resveratrol has been shown to cause SIRT1 to cause migration of FOXO transcription factors to the nucleus [57] which stimulates FOXO3a transcriptional activity [58] and it has been shown to enhance the sirtuin-catalyzed deacetylation (activity) of FOXO3a. MnSOD is known to be a target of FOXO3a, and MnSOD expression is strongly induced in cells overexpressing FOXO3a [59].
Resveratrol interferes with all three stages of carcinogenesis - initiation, promotion and progression. Experiments in cell cultures of varied types and isolated subcellular systems in vitro imply many mechanisms in the pharmacological activity of resveratrol. These mechanisms include modulation of the transcription factor NF-kB,[60] inhibition of the cytochrome P450 isoenzyme CYP1A1[61] (although this may not be relevant to the CYP1A1-mediated bioactivation of the procarcinogen benzo(a)pyrene[62]), alterations in androgenic[63] actions and expression and activity of cyclooxygenase (COX) enzymes. In some lineages of cancer cell culture, resveratrol has been shown to induce apoptosis, which means it kills cells and may kill cancer cells.[63][64][65][66][67][68] Resveratrol has been shown to induce Fas/Fas ligand mediated apoptosis, p53 and cyclins A, B1 and cyclin-dependent kinases cdk 1 and 2. Resveratrol also possesses antioxidant and anti-angiogenic properties.[69][70]
Resveratrol was reported effective against neuronal cell dysfunction and cell death, and in theory could help against diseases such as Huntington's disease and Alzheimer's disease.[71][72] Again, this has not yet been tested in humans for any disease.
Research at the Northeastern Ohio Universities College of Medicine and Ohio State University indicates that resveratrol has direct inhibitory action on cardiac fibroblasts and may inhibit the progression of cardiac fibrosis.[73]
According to Patrick Arnold it also significantly increases natural testosterone production from being both a selective estrogen receptor modulator[74][75] and an aromatase inhibitor.[76][77]
In December, 2007, work from Irfan Rahman's laboratory at the University of Rochester demonstrated that resveratrol increased intracellular glutathionelevels via Nrf2-dependent upregulation of gamma-glutamylcysteine ligase in lung epithelial cells, which protected them against cigarette smoke extract induced oxidative stress. [78]
Pharmacokinetics of Resveratrol
Resveratrol bioavailability depends on its conjugate forms: glucuronate and sulfonate, but most in vitro studies use the aglycone form of resveratrol ('aglycone' means without a sugar molecule attached, as in the figure in this article). In humans[79] [80] and rats,[81] [82] [83]resveratrol rapidly undergoes conjugation resulting in less than 5% of the oral dose being observed as free resveratrol in blood plasma. The effect of conjugation on efficacy is debated.[84][85] The studies[80] [81] show rats have 72% more free resveratrol and 6 times more of a glucuronide form as humans for a dose of about 60 mg/kg. The most abundant conjugates in humans, rats, and mice are trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate.[86] Walle suggests sulfate conjugates are the primary source of activity[79], Wang et al suggests the glucuronides,[87] and Boocock et al also emphasized the need for further study of the effects of the metabolites including the possibility of deconjugation to free reservatrol inside cells.
In humans, Walle et al reported at least 70% absorbed and 99% is quickly metabolized to conjugates using 25mg doses. Half life of resveratrol and its metabolites was 9 hours. 73% was excreted in urine and feces after 12 hours. [79] Boocock et al reported 3% of the total metabolites in the blood plasma of humans was free resveratrol, measured as area under the concentration curve. Half life was 4 to 11 hours for the various metabolites. [80]
A study on rats given an oral dose of 50 mg/kg showed resveratrol and its metabolites have an initial apparent half life of 8 minutes but a "terminal" half life of 1.5 hours in blood plasma. The free resveratrol was 46 times less than the glucuronide form (measured as area under the concentration curve).[81]
When trying to calculate an equivalent dose for humans, it is not accurate to use the mg dose per body weight method as was commonly reported in the media when the life-extension resveratrol studies came out.[88] This method leads to much larger quantities than is accurate because larger animals have a slower metabolic rate for their weight. The comparative dosage should be based on mg per Calories consumed per day. If the Calories consumed per day are not known, it is estimated from the body weight.[89] This results in the formula: (human dose/kg) = (animal dose mg/kg) x (animal kg/human kg)^(1-P) where P=2/3 is used by convention to give a larger margin of safety for FDA pharmaceutical and EPA toxicology uses, but P=3/4 is more accurate.[90] [91] The absorption, metabolism, and excretion is very different in different species so that even adjusting for metabolic rate is not very accurate. For example, as mentioned in the absorption section, rats have much more free resveratrol and glucuronide forms in their blood than humans for a given dose, eventhough the metabolic rate adjustment implies they should have much less.
Related compounds
Scientists are also studying three other synthetic compounds based on resveratrol which more effectively activate the same biological mechanism.[92]
The compound called SRT 1720 seems to be 1000 times more effective than resveratrol, although it only increases SIRT1 activation by 6 times. No data has been publicly produced by Sirtris regarding this difference in SIRT1 efficiency for the new compound.[93]
A study by Professor Roger Corder has identified a particular group of polyphenols, known as oligomeric procyanidins, which they believe offer the greatest degree of protection to human blood-vessel cells. These are found in greatest concentration in European red wines from certain areas, which correlates with longevity in those regions. This new data may impact the supplement market.[94] Because they are present in red wine in more significant quantities, they could offer an alternate explanation of the French paradox.
About Pterostilbene
Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene) is a stilbenoid chemically related to resveratrol. In plants, it serves a defensive phytoalexin role. Pterostilbene is found in almonds, various Vaccinium berries (including blueberries,, grape leaves and vines.As pterostilbene is absent on the list of compounds with GRAS by the US Food and Drug Administration (FDA), its safety for consumption remains unproven as of 2019. (US Food and Drug Administration, Food Ingredient and Packaging Inventories. 6 February 2019). Pterostilbene differs from resveratrol by exhibiting increased bioavailability (80% compared to 20% in resveratrol) due to the presence of two methoxy groups which cause it to exhibit increased lipophilic and oral absorption.
See: Resveratrol and Pterostilbene Exhibit Anticancer Properties Involving the Downregulation of HPV Oncoprotein E6 in Cervical Cancer Cells--"...Results point to a mechanism that may involve the downregulation of the HPV E6 oncoprotein, activation of apoptotic pathways, and re-establishment of functional p53 protein, with pterostilbene showing greater efficacy than resveratrol...."
References
- Farina A, Ferranti C, Marra C (2006). "An improved synthesis of resveratrol". Nat. Prod. Res. 20 (3): 247-52. doi:10.1080/14786410500059532. PMID 16401555.
- Renaud S, Ruf JC (1994). "The French paradox: vegetables or wine". Circulation 90 (6): 3118-9. PMID 7994864.
- LeBlanc, Mark R.. ULTIVAR, JUICE EXTRACTION....
- Lamuela-Raventos, RM (1995). "Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines". J. Agric. Food Chem. (43): 281-283. pubs.acs.org.
- Prokop J, Abrman P, Seligson AL, Sovak M (2006). "Resveratrol and its glycon piceid are stable polyphenols". J Med Food 9 (1): 11–4. doi:10.1089/jmf.2006.9.11. PMID 16579722.
- Bertelli AA, Gozzini A, Stradi R, Stella S, Bertelli A (1998). "Stability of resveratrol over time and in the various stages of grape transformation". Drugs Exp Clin Res 24 (4): 207-11. PMID 10051967.
- See review:Baur JA, Sinclair DA (2006). "Therapeutic potential of resveratrol: the in vivo evidence". Nat Rev Drug Discov 5(6): 493–506. doi:10.1038/nrd2060. PMID 16732220.
- Roy, H., Lundy, S., Resveratrol, Pennington Nutrition Series, 2005 No. 7
- LeBlanc, Mark Rene (2005-12-13). Cultivar, Juice Extraction, Ultra Violet Irradiation and Storage Influence the Stilbene Content of Muscadine Grapes (Vitis Rotundifolia Michx.).
- Gu X, Creasy L, Kester A, et al., Capillary electrophoretic determination of resveratrol in wines. J Agric Food Chem 47:3323-3277, 1999
- Ector BJ, Magee JB, Hegwood CP, Coign MJ., Resveratrol Concentration in Muscadine Berries, Juice, Pomace, Purees, Seeds, and Wines.
- Mozzon, M. (1996). "Resveratrol content in some Tuscan wines". Ital. j. food sci. 8 (2): 145–152. Chiriotti, Pinerolo, ITALIE.
- Higdon, Jane (2005). Resveratrol. Oregon State University. The Linus Pauling Institute Micronutrient Information Center.
- Resveratrol. The Peanut Institute (1999).
- Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB (2002). "An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine.". J. Agric. Food Chem. 50 (3): 431-5. PMID 11804508.
- Lyons MM, Yu C, Toma RB, et al (2003). "Resveratrol in raw and baked blueberries and bilberries". J. Agric. Food Chem.51 (20): 5867-70. doi:10.1021/jf034150f. PMID 13129286.
- Rimas, Andrew. "MOLECULAR BIOLOGIST DAVID SINCLAIR, MEETING THE MINDS", Boston Globe, boston.com,
- Stipp, David. "Can red wine help you live forever?", Fortune magazine, CNN, 2007-01-19, pp. 3.
- "MM2 Group Announces Record Sales of Its Resveratrol Grape Powder", MM2 Group, Earthtimes.org, 2006-11-29.
- Zachary M. Seward (2006-11-30). Quest for youth drives craze for 'wine' pills (htm). The Wall Street Journal.
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. "Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan". Nature. 2003 Sep 11;425(6954):191-6. Epub 2003 Aug 24. PMID 12939617 [1]
- Wood JG, Rogina B, Lavu1 S, Howitz K, Helfand SL, Tatar M, Sinclair D. "Sirtuin activators mimic caloric restriction and delay ageing in metazoans". Nature. 2004 Aug 5; 430(7000):686–689. Epub 2004 Jul 14. PMID 15254550 [2]
- JAN GRUBER et al, "Evidence for a Trade-Off between Survival and Fitness Caused by Resveratrol Treatment of Caenorhabditis elegans" Ann. N.Y. Acad. Sci. 1100: 530–542 (2007).
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. (2007). "Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans.". Mechanisms of ageing and development 128 (10): 546-552. doi:10.1016/j.mad.2007.07.007. PMID 17875315.
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A "Resveratrol Prolongs Lifespan and Retards the Onset of Age-Related Markers in a Short-Lived Vertebrate." Current Biology 2006 Feb 7;16 (3):296-300 PMID 16461283
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. "Resveratrol improves health and survival of mice on a high-calorie diet" Nature 2006 advanced publication
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997). "Cancer chemopreventive activity of resveratrol, a natural product derived from grapes". Science 275 (5297): 218-20. PMID 8985016.
- See review:Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL (2007). "Resveratrol: a review of preclinical studies for human cancer prevention". Toxicol. Appl. Pharmacol. 224 (3): 274–83. doi:10.1016/j.taap.2006.12.025. PMID 17306316.
- Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M (2002). "Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol". Carcinogenesis 23(9): 1531–6. PMID 12189197.
- Saiko P, Horvath Z, Murias M, Handler N, Jaeger W, Erker T, Fritzer-Szekeres M, Szekeres T. "Antitumor Effects of 3,3',4,4',5,5'-Hexahydroxystilbene in hl-60 Human Promyelocytic Leukemia Cells". Nucleosides Nucleotides Nucleic Acids 25 (9). PMID 17065056.
- Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. "Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer". Current Drug Targets (4). PMID 16611030.
- Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ (2005). "Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4'-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells". Int. J. Cancer 115 (2): 194–201. doi:10.1002/ijc.20884. PMID 15688382.
- resveratrolonhumanadipocytebiology,[3]
- Wade, Nicholas (November 16 2006). "Red Wine Ingredient Increases Endurance, Study Shows". New York Times.
- Lagouge M, Argmann C, Gerhart-Hines Z, et al (2006). "Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha". Cell 127 (6): 1109-22. doi:10.1016/j.cell.2006.11.013. PMID 17112576.
- Heredia A, Davis C, Redfield R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J Acquir Immune Defic Syndr. 2000 Nov 1;25(3):246-55. PMID 11115955
- Faith SA, Sweet TJ, Bailey E, Booth T, Docherty JJ. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral research 2006 Jul 14 PMID 16876885
- Palamara AT, Nencioini L, Aquilano K, et al. Inhibition of influenza A virus replication by resveratrol. Journal of Infectious Diseases May 2005 15;191(10):1719–29. PMID 15838800
- Gehm BD, McAndrews JM, Chien P, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. National. Academy of Sciences 1997 Dec 9;94(25):14138-43. PMID 9391166
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000 Oct;141(10):3657-67. PMID 11014220
- Levi, Pasche, Lucchini, Ghidoni, Ferraroni, La Vecchia. Resveratrol and breast cancer risk. European Journal of Cancer Prevention 14(2):139–142, April 2005.[4]
- Garvina, Öllingerb, Dabrosin.Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Letters Volume 231, Issue 1, 8 January 2006, Pages 113-122.[5]
- More resveratrol information, What is Resveratrol?.[6]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE (2007). "Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent". Cancer Epidemiol. Biomarkers Prev. 16 (6): 1246–52. doi:10.1158/1055-9965.EPI-07-0022. PMID 17548692.
- Cell, Vol 127, 1109–1122, 15 December 2006; Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α
- Kaeberlein M et al. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004 Sep;2(9):E296 calorie restriction in yeast. PLoS Biol. 2004 Sep;2(9):E296. PMID 15328540
- Kaeberlein et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005 Apr 29; 280(17):17038-45. PMID 15684413.
- Ellen L. Robb, Melissa M. Page, Brent E. Wiens, Jeffrey A. Stuart, Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD, Biocehm and Biophys Res Comm, 2008
- Zsolt Radák, Free Radicals in Exercise and Aging, 2000, p39
- L.A. MacMillan-Crow, D.L. Cruthirds, Manganese superoxide dismutase in disease, Free Rad. Res. 34 (2001) 325-336.
- Mamta Kanwar, Pooi-See Chan, Timothy S. Kern, and Renu A. Kowluru, The Role of Manganese Superoxide Dismutase in the Growth of Pancreatic Adenocarcinoma, Cancer Research 63, 1297-1303, March 15, 2003
- "Mounting Evidence Shows Red Wine Antioxidant Kills Cancer", Department of Radiation Oncology, University of Rochester Medical Center.,
- J. Sun, D. Folk, T.J. Bradley, J. Tower, Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster, Genetics 161 (2002) 661–672
- D. Hu, P. Cao, E. Thiels, C.T. Chu, G.Y. Wu, T.D. Oury, E. Klann, Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase, Neurobiol. Learn. Mem. 87 (2007) 372–384.
- G M. Stefani, A. Markus, R.C.Y. Lin, M. Pinese, I.W. Dawes, B.J. Morris, The effect of resveratrol on a cell model of human aging, Annals N.Y. Acad. Sci. 1114 (2007) 407-418.
- A. Brunet, L.B. Sweeney, J.F. Sturgill, K.F. Chua, P.L. Greer, Y. Lin, H. Tran, S.E. Ross, R. Mostoslavsky, H.Y. Cohen, L.S. Hu, H.-L. Cheng, M.P. Jedrychowski, S.P. Gygi, D.A. Sinclair, F.W. Alt, M.E. Greenberg, Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase, Science 303 (2004) 2011–2015.
- G.J.P.L. Kops, T.B. Dansen, P.E. Polderman, I. Saarloos, K.W.A. Wirtz, P.J. Coffer, T.-T. Huang, J.L. Bos, R.H. Medema, B.M.T. Burgering, Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress, Nature 419 (2002) 316-321.
- Leiro J, Arranz JA, Fraiz N, SanmartÃn ML, Quezada E, Orallo F (2005). "Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling". Int. Immunopharmacol. 5(2): 393-406. doi:10.1016/j.intimp.2004.10.006. PMID 15652768.
- Chun YJ, Kim MY, Guengerich FP (1999). "Resveratrol is a selective human cytochrome P450 1A1 inhibitor". Biochem. Biophys. Res. Commun. 262 (1): 20-4. doi:10.1006/bbrc.1999.1152. PMID 10448061.
- Schwarz D, Roots I (2003). "In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1". Biochem. Biophys. Res. Commun. 303 (3): 902-7. PMID 12670496.
- Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA (October 18 2006). "Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines". Journal of Andrology. PMID 17050787.
- Faber AC, Chiles TC (Dec 2006). "Resveratrol induces apoptosis in transformed follicular lymphoma OCI-LY8 cells: Evidence for a novel mechanism involving inhibition of BCL6 signaling". International Journal of Oncology 29 (6). PMID 17088997.
- Riles WL, Erickson J, Nayyar S, Atten MJ, Attar BM, Holian O (21 Sep 2006). "Resveratrol engages selective apoptotic signals in gastric adenocarcinoma cells". World Journal of Gastroenterology 12 (35). PMID 17007014.
- Sareen D, van Ginkel PR, Takach JC, Mohiuddin A, Darjatmoko SR, Albert DM, Polans AS (September 2006). "Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells". Investigative Ophthamology & Visual Science 47 (9). PMID 16936077.
- Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY (Aug 2006). "Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells" 5 (8). PMID 16928824.
- Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N (May 2006). "Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins". Molecular Cancer Therapeutics 5 (5). PMID 16731767.
- Cao Y, Fu ZD, Wang F, Liu HY, Han R (2005). "Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants". Journal of Asian natural products research 7 (3): 205-13. doi:10.1080/10286020410001690190. PMID 15621628.
- Hung LM, Chen JK, Huang SS, Lee RS, Su MJ (2000). "Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes". Cardiovasc. Res. 47 (3): 549-55. PMID 10963727.
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in C. elegans and mammalian neurons. Nature Genetics 2005 ; 4 : 349-50. PMID 15793589
- Philippe Marambaud et al., Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. Journal of Biological Chemistry 2005 ; 280(45):37377-82 PMID 16162502
- Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol2005 Mar;288(3):H1131-8. PMID 15498824
- Juan ME, Gonzalez-Pons E, Munuera T, Ballester J, RodrÃguez-Gil JE, Planas JM (2005). "trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats.". J Nutr. 135 (4): 757-60. PMID 15795430.
- Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM (2001). "Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models.". Cancer Res. 61 (20): 7456-63. PMID 11606380.
- Wang Y, Lee KW, Chan FL, Chen S, Leung LK (2006). "The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells.". Toxicol Sci. 92 (1): 71-7. PMID 16611627.
- Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C (2004). "Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels.". J Clin Endocrinol Metab 89 (3): 1174-80. PMID 15001605.
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I (2007). "Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells.". Am J Physiol Lung Cell Mol Physiol.. doi:10.1152. PMID 18162601.
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK (2004). "High absorption but very low bioavailability of oral resveratrol in humans". Drug Metab. Dispos. 32 (12): 1377–82. doi:10.1124/dmd.104.000885. PMID 15333514.
- David J. Boocock, et al (2007). "Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent". Cancer Epidemiology Biomarkers & Prevention 16 (6): 1246-1252. doi:10.1158/1055-9965.EPI-07-0022.
- Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP (2002). "Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model". J. Pharmacol. Exp. Ther. 302 (1): 369-73. PMID 12065739.
- Distribution of [3H]trans-resveratrol in rat tissu...[Br J Nutr. 2006] - PubMed Result
- Bioactivity and metabolism of trans-resveratrol or...[Mol Nutr Food Res. 2005] - PubMed Result
- Scientists question power of resveratrol supplements.
- Goddard, Ian. Resveratrol Bioavailability Analysis. sci.life-extension.
- Human, rat, and mouse metabolism of resveratrol. [Pharm Res. 2002] - PubMed Result
- Resveratrol glucuronides as the metabolites of res...[J Pharm Sci. 2004] - PubMed Result
- Dose translation from animal to human studies revi...[FASEB J. 2008] - PubMed Result
- Whitfield, John "IN THE BEAT OF A HEART", 2006 Joseph Henry Press, p56 http://books.nap.edu/openbook.php?record_id=11634&page=56
- Draft Guidance for Industry and Reviewers: Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers
- "Air Toxics and Risk Assessment" By Edward J. Calabrese, Elaina M. Kenyon, 1991 CRC Press
- Scientists Develop Life-Extending Compounds
- Scientists Locate Revved Up Chemical That Mimics Red Wine
- R. Corder et al., Oenology: Red wine procyanidins and vascular health., Nature vol. 444, p. 566; 30 November 2006
Anti-Aging and Senolytics Home Page
- What is Anti-Aging Medicine
- What is Senescence?
- What are Senolytics?
- About Caloric Restriction
- Mtor and Rapamycin
- The IKK/NF-κB signaling pathway in aging
- Exercise and Anti-Aging
- Meditation and Anti-Aging
SENOLYTIC AND ANTI-AGING MOLECULES
RAPAMYCIN ---The mechanistic target of rapamycin (mTOR) pathway has a central role in cell activation...
METFORMIN -- The diabetes drug metformin used by some for anti-aging may diminish benefits of aerobic exercise...
QUERCETIN-- AND WITH DASATINIB--The senolytic cocktail, dasatinib plus quercetin, which causes selective elimination of senescent cells...
FISETIN--Of the 10 flavonoids tested, fisetin was the most potent senolytic...
EGCG- The most active component of green tea....
NAD BOOSTERS --'...The cells of the old mice were indistinguishable from the young mice, after just one week of treatment...
SULFORAPHANE-- An isothiocyanate present in cruciferous vegetables activates antioxidant and anti-inflammatory responses by ...
UROLITHIN --Metabolite of Pomegranate compound with anti-aging effects passes human trial...
MITO-Q -- A water soluble fomr of CoQ10 that has excellent absorption and high bioavailability...
HONOKIOL - A bioactive natural product derived from Magnolia Bark have demonstrated ...
CURCUMIN AND ANALOGS -Recent research is focused on the design and synthesis of curcumin analogs as antiproliferative and anti-inflammatory agents...
BERBERINE --berberine has recently been reported to expand life span in Drosophila melanogaster, and attenuate premature cellular senescence
N-ACETYL-CYSTINE (NAC)--"...pretreatment with NAC increased glutathione levels in the older cells and largely helped offset that level of cell death..."
PIPERLONGUMINE - A natural product from the Long pepper with high bioavailability...
RESVERATROL AND PTEROTSILBINE -- Pterostilben chemically similar to resveratrol bute differs from resveratrol by exhibiting increased bioavailability (80% compared to 20% in resveratrol)
SPERMIDINE--Spermidine delays aging in humans ...
ALLICIN -- Allicin is a compound produced when garlic is crushed or chopped. ...
VITAMIN D3 -- Production of the active forms of Vitamin D are reduced by 50% as a result of an age-related decline
VITAMIN K-- evidence suggests vitamin K has an anti-inflammatory action
TOCOTRIENOL(AND WITH QUERCETIN) --Tocotrieniols have been found to exert a synergistic antitumor effect on cancer cells when given in combination....
HSP-90 INHIBITORS --As a novel class of senolytics
The Cannabidiol Molecule
Cannabidiol (CBD is the major non-psychoactive component of Cannabis and is being looked at by major drug and consumer companies for various medical and social uses.