How does Paxlovid work on SARS-CoV-2?
Paxlovid is a combination of two drugs: the experimental antiviral PF-07321332; and the antiretroviral drug Ritonavir that is used along with other medications to treat HIV/AID. An interim analysis of the trial data showed that the drug was found to reduce the risk of hospitalization or death by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19.
If approved or authorized, PAXLOVID™, which originated in Pfizer’s laboratories, would be the first oral antiviral of its kind, a specifically designed SARS-CoV-2-3CL protease inhibitor. see Pfizer Press Release
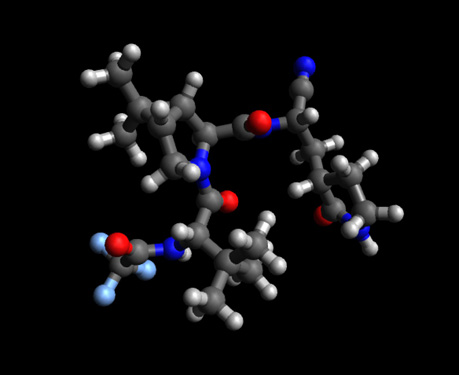
PF-07321332 Molecule -C23H32F3N5O4---PubChem CID 155903259
PF-07321332 (above) is an antiviral drug developed by Pfizer which acts as an orally active The 3C like protease (3CLpro) inhibitor. It is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the enzyme. Note: The 3C-like protease (3CLpro) or Mpro, formally known as C30 Endopeptidase, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.The Enzyme Commission refers to this family as SARS coronavirus main proteinase (Mpro) see: 3C-like protease
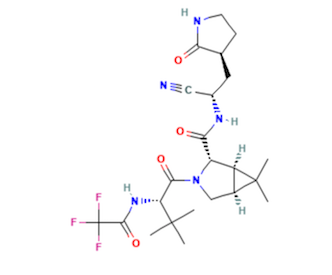
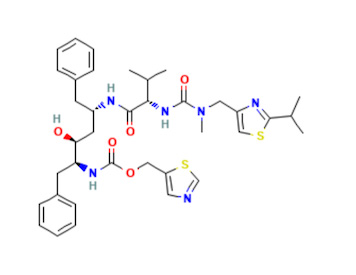
Shown above left: PF-07321332, the primary anti-viral drug in Paxlovid; Shown right: Ritonavir
PF-07321332, a protease inhibitor ,is designed to block replication of SARS-CoV-2. It is co-administration with antiretroviral ritonavir which is best known as an HIV treatment in HAART (highly active antiretroviral therapy).Ritonavir is used to slow the breakdown of PF-07321332, increasing its duration of action.
Pfizer chairman and CEO Albert Bourla calls the combination therapy "a real game changer".
Molecular Mechanism
"...Paxlovid is an oral antiviral drug known under the codename PF-07321332 and administered in combination with a low 100-mg dose of ritonavir (ritonavir). PF-07321332 is a reversible covalent inhibitor of 3C-like protease (3CLpro), an enzyme needed by coronavirus for replication. PF-07321332 blocks the cysteine residue in 3CLpro, thereby inhibiting its activity at the proteolysis stage. Because 3CLpro is responsible for the cleavage of coronavirus polyproteins 1a and 1b, which contain 3CLpro itself, a papain-like protease (PLpro), and 14 other nonstructural proteins, they all cannot be released to perform their functions, resulting in inhibition of viral replication.
Ritonavir is a booster (enhancer) of PF-07321332. Ritonavir inhibits cytochrome P450 3A4 (CYP3A4), a hepatic enzyme that metabolizes xenobiotics, meaning it deactivates drugs. In other words, ritonavir increases the bioavailability of PF-07321332 by helping to slow down the metabolism of this molecule so that it retains its activity in the body for a longer time and in higher concentrations...." see Paxlovid: Clinical Efficacy and Safety of Coronavirus Treatment
A 5-day course of treatment involves taking 20 tablets of Paxlovid and 10 capsules of ritonavir.
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick
Shown above left: PF-07321332, the primary anti-viral drug in Paxlovid; Shown right: Ritonavir
The catalytic residues (His41 --Red, Cys145 ---Yellow)."... The zoom-in view of the substrate-binding pocket. PF-07321332 shows a covalent bond between C145 and PF-07321332. The Sγ atom of the catalytic C145 forms a 1.8-Å C-S covalent bond with the nitrile carbon of PF-07321332...." See: (2021) Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332-- for more details.
Above: 7vh8.pdb: --Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332--(2021) Protein Cell --Yao Zhao, Chao Fang and Qi Zhang et.al...
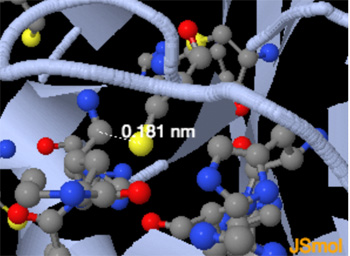
Image above from using zoom with the Jsmol script (above) and showing catalytic site -- C145 forming a 1.8-Å C-S covalent bond with the nitrile carbon of PF-07321332.
Molecular Modeling and Molecular Dynamices Studies
Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332
An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19
Insights into the Binding and Covalent Inhibition Mechanism of PF-07321332 to SARS-CoV-2 Mpro
"...We found that the catalytic triad Cys145–His41–Asp187 of SARS-CoV-2 Mpro plays important role in the activation of PF-07321332 covalent inhibitor, which renders the deprotonation of Cys145 and, thus, facilitates further reaction...."
Discovery of SARS-CoV-2 Mpro peptide inhibitors from modelling substrate and ligand binding†
Clinical Trial for Paxlovid
Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study- PAXLOVID™ (PF-07321332; ritonavir) was found to reduce the risk of hospitalization or death by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19
- In the overall study population through Day 28, no deaths were reported in patients who received PAXLOVID™ as compared to 10 deaths in patients who received placebo
- Pfizer plans to submit the data as part of its ongoing rolling submission to the U.S. FDA for Emergency Use Authorization (EUA) as soon as possible
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules