Why is water such a good solvent?
Water is called the "universal solvent" because it dissolves more substances than any other liquid -- why is that the case.
A water molecule is formed when two atoms of hydrogen bond covalently with an atom of oxygen. In a covalent bond electrons are shared between atoms. In water the sharing is not equal. The oxygen atom attracts the electrons more strongly than the hydrogen. This gives water an asymmetrical distribution of charge. Molecules that have ends with partial negative and positive charges are known as polar molecules. It is this polar property that allows water to separate polar solute molecules and explains why water can dissolve so many substances.
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick
To Rotate the Molecule--->Left Click and Drag
To Zoom-->>Left Click + hold Shift button and Drag Vertically
Jsmol Menu --->>Right-Click
Water is a good solvent due to its polarity.
The solvent properties of water are vital in biology, because many biochemical reactions take place only within aqueous solutions
When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute. The partially negative dipoles of the water are attracted to positively charged components of the solute, and vice versa for the positive dipoles.
An example of an ionic solute is table salt. The dipole forces of water can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and, thus, dissolve it.
Liquid water has a partially ordered structure in which hydrogen bonds are constantly being formed and breaking up.
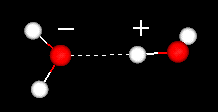
The strong hydrogen bonds also give water a high cohesiveness and, consequently, surface tension. This is evident when small quantities of water are put onto a nonsoluble surface and the water stays together as drops.
What is the distance in angstroms for the hydrogen bond shown above?
------>space fill/cpk -------->stick ----> ball-and-stick
How to measure distance
Label Atoms first
Style -- > Labels -- > with atom Name
Then measure distance
Right mouse button -->Measurements -->
Double click on hydrogen (H31) then drag to oxygen (O28) -- double click again
How to measure angles
Double click on first atom, once on middle atom, twice on atom three.
Check your measurements below
1) What is the distance of the hydrogen bond show?
Please enter your answer in the space provided:
2) What is the measure of the HOH angle in a single molecule of water?
3)What is distance between the oxygen atoms in a water dimer?
Test your Understanding:
Molecules of Life Resources
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?