What is the structure of the Carbyne molecule?In organic chemistry, carbyne is often a 'general term' for any compound whose molecular structure includes an electrically neutral carbon atom with three non-bonded electrons, connected to another atom by a single bond. The chain of carbon atoms is held together by either double or alternating single and triple atomic bonds. That makes it a true one-dimensional material, unlike atom-thin sheets of graphene. Carbon chains are traditionally classified as cumulene (monatomic chains with double bonds, = C = C = ) or polyyne (dimerized chains with alternating single and triple bonds, −C≡C−). A polyyne is any organic compounds with alternating single and triple bonds; that is, (−C≡C−) n with n greater than 1. The simplest example is diacetylene or buta-1,3-diyne, H−C≡C−C≡C−H. Linear acetylenic carbon (also referred to as carbyne) is an allotrope of carbon that has the chemical structure (−C≡C−) n , with alternating single and triple bonds would thus be the ultimate member of the polyyne family. A cumulene is a hydrocarbon with three or more cumulative (consecutive) double bonds. A member of this compound class is butatriene (which is also called simply cumulene), H2C=C=C=CH2. Unlike most alkanes and alkenes, cumulenes tend to be rigid, which makes them appealing for molecular nanotechnology. Cumulenes are found in regions of space where hydrogen is rare. 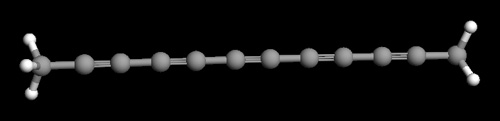
JPEG image of 12 carbon chain carbyne molecule containing alternating carbon single and triple bonds. The image was created using the free software package ArgusLab.
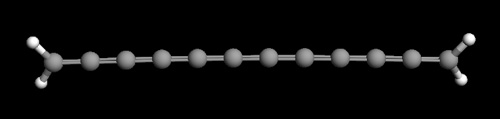
JPEG image of 12 carbon chain carbyne molecule containing all carbon double bonds. The image was created using the free software package ArgusLab.
Note: All molecules on this page were built using Argus Lab 4.01 (see free molecular modeling software). The structures were Geometry Optimized in Argus Lab using the semiempirical method (AM1 Hamiltonian). BOND LENGTHS IN CARBON CHAINS The bond lengths of carbon chains characterizes the type of bond between the carbons, For example alkanes have a bond length of 1.51 ˚A , alkenes 1.33 ˚A and alkynes 1.19 ˚A. This however is not the case with carbynes (See below) To measure bond lengths with Jmol -- double click with left mouse button twice on first atom, then drag cursor to second atom and double click. -------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick Try this: Rotate the Carbyne molecule to see the alternating single and triple bonds (Hold the left mouse button down over the java applet image and move the mouse to rotate the graphite molecule). [Carbyne show with alternating carbon single and triple bonds using the Jsmol] -------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick Try this!!
Rotate the Carbyne molecule to see the consecutive double bonds. [Carbyne shown with alternating carbon double bonds using the Jsmol]
What is the difference between Graphene and Carbyne?Graphene is a one-atom-thick planar sheet of sp2-bonded carbon atoms. Graphene is the basic structural element of some carbon allotropes including graphite, carbon nanotubes, and fullerenes. The Nobel Prize in Physics for 2010 was awarded to Andre Geim and Konstantin Novoselov "for groundbreaking experiments regarding the two-dimensional material graphene". For a more detailed description of graphene see our graphene 3D molecule page.
Carbyne (Linear acetylenic carbon) has been in the news lately as a structure that is possibly the worlds strongest material that has great potential if anyone can make it in bulk. Carbyne is basically a chain of single carbon atoms, but having twice the tensile strength of graphene , and three times the tensile stiffness of diamond (1,2). The chemical structure is repeating chain with alternating single and triple bonds --- ...Because it is just a single strand of molecules it is often referred to as "one-dimensional" structure. Readings and References All-carbon sp-sp2 hybrid structures: Geometrical properties, current rectification, and current amplification --Scientific Reports Sept. 2013 Gaining knowledge of single carbon chains --Radboud University Nijmegen Sept. 2011 The [18] all-carbon molecule: cumulene or polyacetylene --J. Am. Chem. Soc., 1991. |
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules
