Why is Carbon Monoxide so Dangerous?Carbon monoxide (chemical formula CO) is a colorless, odorless, flammable and highly toxic gas. It is a major product of the incomplete combustion of carbon and carbon-containing compounds. Oxygen is a nonmetallic element constituting 21 percent of the atmosphere by volume. Oxygen is also found in many compounds such as water and iron ore. It combines with most elements, is essential for plant and animal respiration, and is required for nearly all combustion.
OXYGEN 2 MOLECULE
-------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick CARBON MONOXIDE (CO) MOLECULE
-------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick
Hemoglobin is a protein molecule with 4 protein sub-units (2 alphas and 2 betas) Carbon monoxide binds very strongly to the iron atoms in hemoglobin, the principal oxygen-carrying compound in blood. The affinity between CO and hemoglobin is 200 times stronger than the affinity between hemoglobin and oxygen. When CO binds to the hemoglobin it cannot be released nearly as readily as oxygen would be. The preferential binding of carbon monoxide to heme iron is the main reason for carbon monoxide poisoning.
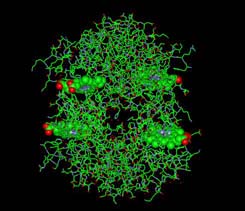
IMAGE OF IRON IN HEME BOUND TO OXYGEN In the lungs oxygen must enter the blood A small amount of oxygen dissolves directly in the serum, but 98.5% of the oxygen is carried by hemoglobin Shown here is one of the four subunits of the hemoglobin molecule. A molecule of oxygen (O2) is shown bound to Fe. The Fe2+ ion is coordinated to 4 N's on the pyrrole ring, The 5th ligand is a proximal His (the 8th amino acid on helix F). Oxgen is shown bound to the 6th coordination site pulling the Fe into the plane of the ring.
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick In the lungs oxygen must enter the blood A small amount of oxygen dissolves directly in the serum, but 98.5% of the oxygen is carried by hemoglobin Shown here is one of the four subunits of the hemoglobin molecule. A molecule of oxygen (O2) is shown bound to Fe. The Fe2+ ion is coordinated to 4 N's on the pyrrole ring, The 5th ligand is a proximal His (the 8th amino acid on helix F). Oxgen is shown bound to the 6th coordination site pulling the Fe into the plane of the ring. FROM PDB FILE: 1A4F HAEMOGLOBIN IN THE OXY FORM
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick IMAGE OF IRON IN HEME BOUND TO CARBON MONOXIDE Carbon monoxide also binds coordinately to heme iron atoms in a manner similar to that of oxygen, but the binding of carbon monoxide to heme is much stronger than that of oxygen. The preferential binding of carbon monoxide to heme iron is the main reason for carbon monoxide poisoning. CO binds to the same heme Fe atoms that O2 binds to. CO displaces oxygen from hemoglobin because it has a 200X greater affinity for hemoglobin. What is a treatment for CO poisoning? --Move the victim to fresh air immediately. Breathing pure O2 can give faster removal of CO FROM PDB FILE: 1GCW 2-D images are all courtesy of Wikipedia. |
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules