How does anti-viral Molnupiravir (covid-19 drug) work?
Molnupiravir (development codes MK-4482 and EIDD-2801) is an oral antiviral drug. The drug was developed at Emory University by the university's drug innovation company, Drug Innovation Ventures at Emory (DRIVE). It was then acquired by Miami-based company Ridgeback Biotherapeutics, who later partnered with Merck & Co. to develop the drug further. The drug was originally developed to treat influenza. Molnupiravir is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication. Molecular MechanismAbstract: "...Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. Molnupiravir increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans. Here, we establish the molecular mechanisms underlying molnupiravir-induced RNA mutagenesis by the viral RNA-dependent RNA polymerase (RdRp). Biochemical assays show that the RdRp uses the active form of molnupiravir, β-d-N4-hydroxycytidine (NHC) triphosphate, as a substrate instead of cytidine triphosphate or uridine triphosphate. When the RdRp uses the resulting RNA as a template, NHC directs incorporation of either G or A, leading to mutated RNA products. Structural analysis of RdRp–RNA complexes that contain mutagenesis products shows that NHC can form stable base pairs with either G or A in the RdRp active center, explaining how the polymerase escapes proofreading and synthesizes mutated RNA. This two-step mutagenesis mechanism probably applies to various viral polymerases and can explain the broad-spectrum antiviral activity of molnupiravir...." source Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis
Molnupiravir is the orally bioavailable 5′-isobutyrate prodrug of a direct-acting antiviral ribonucleoside analog, β-D-N4-hydroxycytidine, or EIDD-1931. Molnupiravir is cleaved in the plasma to liberate EIDD-1931. EIDD-1931 is phosphorylated intracellularly to its corresponding 5′-triphosphate, the active antiviral agent, by host kinases [13, 14]. EIDD-1931 inhibits replication of multiple RNA viruses, and its antiviral activity was verified in animal models of influenza, various coronaviruses, respiratory syncytial virus (RSV), Venezuelan equine encephalitis virus (VEEV), Chikungunya, and Ebola virus infection [15,16,17,18]. from Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2 -------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick Molnupiravir (left) can swap between two forms (tautomers), one of which mimics cytidine (C) and the other of which mimics uridine (U). NHC-TP (Hydroxycytidine 5′-triphosphate (also called EIDD-1931 5′-triphosphate or NHC-TP) is not recognized as an error by the virus' proofreading exonuclease enzymes, which can replace mutated nucleases with corrected versions. When the viral RNA polymerase attempts to copy RNA containing molnupiravir, it sometimes interprets it as C and sometimes as U. This causes more mutations in all downstream copies than the virus can survive, an effect called viral error catastrophe or lethal mutagenesis. see Wikipedia Molnupiravir
Below: 7OZU:pdb file --SARS-CoV-2 RdRp with Molnupiravir/ NHC in the template strand base-paired with A Green shows Molnupiravir -- Yellow shows A15 Adenine Left Jsmol for pdb file 70zu ; right shows RNA strand with M in Green hydrogen bonded to A15 in Yellow
7OZU:pdb file --SARS-CoV-2 RdRp with Molnupiravir/ NHC in the template strand base-paired with A About RdRpRNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template. read more about RdRp on Wikipedia In the presence of NTPs and MTP, M nucleotides can be incorporated by SARS-CoV-2 RdRp instead of C or U (cytidine triphosphate or uridine triphosphate) into the negative-strand genomic (−gRNA) or subgenomic RNA (−sgRNA) during copying of the positive-strand genomic RNA template (+gRNA).
|
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules
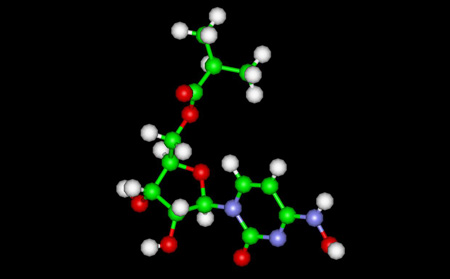
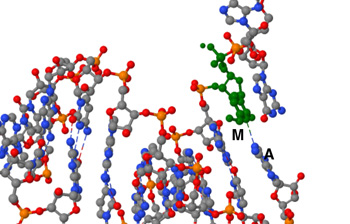 Molnupiravir/NHC (shown in Green) hydrogen bonded to A15 (M-A)
Molnupiravir/NHC (shown in Green) hydrogen bonded to A15 (M-A)