Why is simple soap most effective in destroying coronoavirus -- SARS-CoV-2?
Simple Soap is still the Best --- Hand Sensitizer, while generally effective, is still not the gold standard that soap and water is, according to the CDC, and is considered an acceptable alternative only if there is no access to soap and running water. In addition alcohol-based hand sanitizer must contain at least 60% alcohol to be effective.
Soap directly attacks the virus by disrupting the lipid bilayer membrane of the virus, much like soap and water removes grease from dishes. Running a greasy plate under cold water will not remove the grease. To clean that plate well you need good sop suds and rinsing after will warm water.
Where is the lipid bilayer in the virus... See the image below. The coronavirus contains several proteins embedded within the virus membrane coat. The lipid coat is shown in grey,
Coronavirus structure showing proteins embedded in bilayer lipid membrane
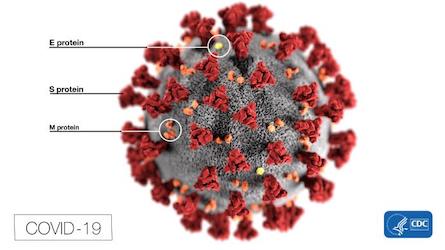
Surface proteins shown above are embedded in the lipid bilayer membrane
Spike, or S protein, is made early in infection. One part of it, S1, grabs a receptor molecule --receptor-binding domain (RBD)-- sticking out of a host cell and another part (S2) fuses to the cell membrane. Three copies of the S protein form each spike. SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2). The S protein serves as a target for development of antibodies, entry inhibitors and vaccines.
Membrane (M) glycoprotein lies beneath the spikes, where it shapes mature viral particles and binds the inner layers. Lipid (fat) is borrowed from host cell membranes during past infections.
Envelope (E) glycoproteins control the assembly, release, and infectivity of mature viruses.
How does the virus get into cells?
The virus attaches to cells by binding the Spike (S-protein) to ACE2 receptor binding domain (RBD). One part of it, S1, grabs a receptor molecule --receptor-binding domain (RBD)-- sticking out of a host cell and another part (S2) fuses to the cell membrane.
The bilayer lipid membrane of coronavirus
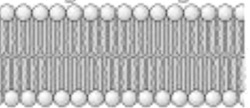
The coronavirus basically has a a layer of fat (lipid bilayer membrane) protecting the virus from the outside world. This is what hold virus together. Dissolve apart the lipid membrane and virus will fall apart and become inactive. The spike protein is attached to the virus membrane via the S2 segment (See image below)
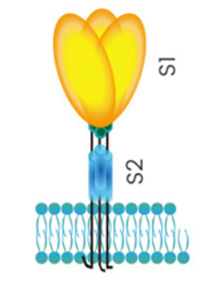
Original Image Source: BioConnect-Life Sciences
Why do coronaviruses easily attach to skin?
The skin is an ideal surface for a virus. Skin surface contains proteins and fatty acids from the dead cells.. These molecules interact with the surface of the virus via both hydrogen bonds and "fat-like" hydrophilic interactions, If you then touch a steel surface with a virus particle on it, it will stick to your skin and get transferred onto your hands. If you then touch your face the virus can get transferred from your hands and on to your face.
How does soap work against coronavirus?
What is Soap?
Soaps are mixtures of sodium or potassium salts of fatty acids which can be derived from oils or fats by reacting them with an alkali (such as sodium or potassium hydroxide) at 80°–100 °C in a process known as saponification.
fat + NaOH ---> glycerol + sodium salt of fatty acid
- CH2-OOC-R - CH-OOC-R - CH2-OOC-R (fat) + 3 NaOH ( or KOH)
both heated --->
- CH2-OH -CH-OH - CH2-OH (glycerol) + 3 R-CO2-Na (soap) R=(CH2)14CH3
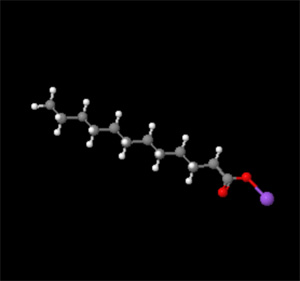
Molecule shown is Sodium Laurate C12H23NaO2
Notice that one end of the molecules is made up of a hydrocarbon chain -- the other end is a very polar structure containing oxygen sodium.
Soap molecules have both properties of non-polar and polar at opposite ends of the molecule.
For 3-D interactive molecule using Jsmol
How does Soap Work?
Nearly all compounds fall into one of two categories: hydrophilic ('water-loving') and hydrophobic ('water-hating'). Water and anything that will mix with water are hydrophilic. Oil and anything that will mix with oil are hydrophobic. When water and oil are mixed they separate. Hydrophilic and hydrophobic compounds just don't mix.
The cleansing action of soap is determined by its polar and non-polar structures in conjunction with an application of solubility principles. The long hydrocarbon chain is non-polar and hydrophobic (repelled by water). The "salt" end of the soap molecule is ionic and hydrophilic (water soluble).
When grease or oil (non-polar hydrocarbons) are mixed with a soap- water solution, the soap molecules work as a bridge between polar water molecules and non-polar oil molecules. Since soap molecules have both properties of non-polar and polar molecules the soap can act as an emulsifier. An emulsifier is capable of dispersing one liquid into another immiscible liquid. This means that while oil (which attracts dirt) doesn't naturally mix with water, soap can suspend oil/dirt in such a way that it can be removed. The soap will form micelles (see below) and trap the fats within the micelle. Since the micelle is soluble in water, it can easily be washed away.
When you mix soap into the water the soap molecules arrange themselves into tiny clusters (called 'micelles'). The water-loving (hydrophilic) part of the soap molecules points outwards, forming the outer surface of the micelle. The oil-loving (hydrophobic) parts group together on the inside, where they don't come into contact with the water at all. Micelles can trap fats in the center.
Sars-CoV-2 Variants
The novel coronavirus SARS-CoV2
B.1.1.7 and B.1.525 UK variant
B.1.427/B.1.429, California QP77P Mutation
B.1.526, NY E484K or S477N Mutations
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- How does soap work?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
Molecules of Life Resources